Our goal was to find the main factor that causes changes in the behaviour of flower of Nymphaea candida, by observing flowers in nature during different photoperiods. The following conclusions were made: (1) The change of solar radiation activity is the main natural factor which causes the rhythmic cycle of Nymphaea candida flower behavior. (2) The changing of average opening point of N. candida flower is an endogenous biological rhythm. (3) The changing of average depth of this species’ flower submergence is a feebly marked exogenous biological rhythm. (4) The average point of N. candida flower openness and the average depth of its submergence are independent of each other. (5) Dependence of N. candida flower behavior on the atmospheric pressure and average air humidity was not found.
Key-words: white water-lily, Nymphaea candida, flower behaviour, continous observations, nature habitat, light intensity.
Most of the processes observed in living organisms have rhythms of an endogenous nature (Bunning, 1931). Many authors indicate necessity of synchronization stimulation for rhythms to appear in plant tissue, as well as the inability of green plant cells’ to synchronize with each other (Wilkins, 1964; Ehmme, 1965; Winfry, 1990). There is another opinion about plant cells’ ability to pass on excitation from one to another in various ways (Stein-Margolina, 1982). Many authors have established the key role of sunlight in the synchronization of plant cell activity (Wilkins, 1964; Ehmme, 1965; Winfry, 1990).
One of the most striking example of circadian rhythms is the behaviour of the white water-lily flower (Nymphaea candida J. et C. Presl.). It is well-known that during clear weather, in the morning, water-lily flowers rise on the water surface and open, when the evening comes they plunge into the water and close (Kerner fon Marylaun, 1891; Velde, 1986; Artamonov, 1989 and others). It is still unknown what causes such behavior. Most authors say that it is caused by light (Kerner fon Marylaun, 1891; Ehmme, 1965; Winfri, 1990). V.I. Artamonov (1989) suggests that flower behavior changes due to the water and air temperature, while B. Suini and D. Gastings (1964) dispute this. It is hypothesized that the change in turgor makes cells change in size and thus controls the degree of openness and the depth of submergence for the flower (Kerner fon Marylaun, 1891). Furthermore, A. Kerner fon Marylaun (1891) and V.A. Stein-Margolina (1982) find that the different growth speed of the inner and outer surfaces of the petals and sepals during flower development, which causes the periodic changes in the degree of flower openness.
There are many different hypothesis, dealing with the determination of the degree of N. candida flower openness change during the day. Some authors believe that this phenomenon is necessary to hold the fertilizer insects inside a flower (Kerner fon Marylaun, 1891) or to "choose" the particular fertilizer species (Wiersema, 1988), to protect pollen from the intrusion of dew (Kerner fon Marylaun, 1891), or to prevent heat loss at cold night (Artamonov, 1989).
Our earlier field research have shown that in the middle region of Western Russia (Udomelski area, Tverskaya district; 58° 15' N, 34° 30' E) during the natural photoperiod, the degree of flower openness and the degree of submergence for the N. candida flower depend on natural 24-hour factors, such as solar radiation and water and air temperature (Volkova et al., 2002). In nature, those factors occur in close connection with each other, which is why it was impossible to find out which one of them was the determinative one. In this research, we tried to find the main factor that causes the change in flower behavior, observing flowers during different photoperiods in natural conditions.
The studies took place in lake Moldino (Udomelski district, Tverskaya region; 58° 15' N, 34° 30' E) for 132 hours from 24 to 30 June 2002 and in lake Tainoe (Republic of Karelia, Loukhski district; 66° 20' N, 33° 20' E) for 64 hours from 29 to 31 July, 2002. Fifteen flowers were observed during the natural photoperiod in Tverskaya region (29 June had 18 hr and 17 min of light time). We also studied flowers in experimental conditions. Five flowering plants were illuminated by magnesium flash for 3 seconds on 28 June (time was 00:00). Another 15 flowering plants were nearly deprived of light for the whole research period. In order to make this possible a chamber of lightproof film had been constructed around those plants. Those parts of a chamber that were on the sunny side and the roof were covered with light reflecting film to prevent flowers from overheating. By periodically checking the temperature inside the chamber, we could see that there was no substantial difference between this value and the air temperature outside. In order to observe our flowers at night we used a lamp with an interference "green" colour filter and with weak light energy. This kind of light does not break the conditions of an experiment because plants do not assimilate it (Kleshnin, 1954).
In Karelia we observed 15 lilies which had no dark phase (29 July had 19 hr and 17 min of light time).
A floating mark with an individual number was attached to each of the flowers’ common peduncle. This kind of marking excludes any kind of confusion in flowers and has no influence on their vital functions. In the course of all our research, we noted each of the flowers’ "average degree of openness" and "average degree of submergence" once every two hours the stage of their development and once every eight hours. All these characteristics were valued by sight using the five-point scale (table 1, 2).
Table 1. Criterions for determination of the point of flower openness and the depth of flower submergence.
| Point | Openness | Submergence |
| 1 | flower is fully closed | Flower is floating on the water surface |
| 2 | flower is almost closed | Flower is submerged for 1/3 of its height |
| 3 | flower is half open | Flower is submerged for 1/2 of its height |
| 4 | flower is almost open | Flower is submerged for 2/3 of its height |
| 5 | flower is fully open | Flower is submerged fully under water |
Table 2. Criterions for determination of flower development stages (accordingly Velde, 1986; Wiersema, 1988)
| Stage name | Flower condition |
| Bud stage | Flower is closed, sepals are close to each other |
| Pistillate stage | Flower opened latterly, anthers are immature, stigmatic disk is dewy |
| Transitional stage | Anthers mature and incline to stigmatic disc. |
| Staminal stage | Stigmatic disc is dry, all anthers are mature, pollen actively products. |
| Terminal stage | the end of blooming; no pollen production, stigmatic disk is dry. |
During each observation, we measured atmospheric pressure, water top layer temperature, and air temperature. In addition, in Tverskaya region we observed the nebulosity value, and with the help of an aspiration psychrometer we registered comparative air moisture and photosyntetically active solar radiation, farther PSR (wavelength interval 400-710 nm; Voskresenskaya, 1965). A colour pyranometer device (model GGO M-80) and a galvanometer device (model GSA-1) (Mahotkina, 1983) were used to measure PSR.
In order to understand the general flower behavior tendency during the given photoperiod, we calculated the "average degree of flower openness" (an arithmetic average for the degree of openness for each flower during one observation) and the "average degree of submergence" (an arithmetic average for degree of submergence for each flower) for each of the photoperiods. Information about light time length was received with the help of the computer program Xephem for astronomic calculations (Downey, 2000). Statistical processing The flower carries out its reproduction functions during pistillate, transitional and staminal stages (Velde, 1986; Wiersema, 1988), and that is why we have chosen to analyze flower behavior during those periods. We visually analyzed the cause-and-effect diagram for the "average degree of submergence", "average degree of flower openness" and "absolute time" (the time which had passed since the beginning of the research) (fig. 1-4, table 3).
We counted the correlation coefficients between "average degree of flower submergence", "average degree of flower openness", atmospheric pressure, top layer water temperature, air temperature, value of nebulosity, comparative air moisture, and PSR. We used Spirman's nonparametric coefficients of rank correlation because data distribution was different from normal. Data processing was possible with the help of STATISTICA package for Windows (StatSoft, Inc., 1999).
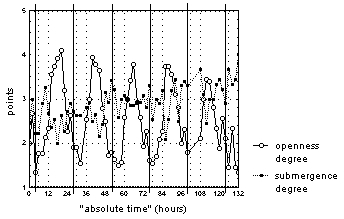
"Average degree of flower openness" and "average degree of flower submergence" for lily flowers correlate with degree of openness and degree of submergence for each of the flowers (R=0,3-0,7). Thus, the general behavior tendency can be identically described with this average exponent. The changes of flower "average degree of openness" had a clear 24-hours period against a gradual arhythmic growth of "medium depth of submergence point" with weak daily rhythms, which is probably connected with the end of the blooming period. Every day flowers were open from 10:00-12:00 until 14:00-16:00 and closed from 18:00-0:00 until 8:00. At the same time, all open flowers were floating on the water surface (fig. 1).
Figure 1. The change of "average degree of flower openness" and "average degree of flower submergence" for N. candida during natural photoperiod in European part of mid-Russia region.

Vertical continuous lines mark 00:00 time for each day of observations.
When analyzing individual flowers’ behavior a large dispersion in time was found for periods when flowers were opened and closed (from 8:00-12:00 until 14:00-22:00). Also, deviations from typical behavior were found for all observed flowers at different stages of development, which did not depend on daytime or registered weather conditions. Nearly 75% of flowers during the day repeatedly submerged fully or halfway into water in open and half-open conditions. Half of the flowers showed rapid change in the depth of submergence point, or they floated on the water surface during the dark day period. It is clear from the description of flower behavior that during the mid-Russia region’s natural photoperiod a weak negative dependence is seen between flower "average degree of openness" and "average degree of submergence". This kind of dependence (R=-0,3... -0,5) was registered only once for 30% of the flowers, which held in this photoperiod.
"Average degree of openness" for flowers was directly proportional (R=0,6) to PSR intensity, while for their "average degree of submergence" inversely proportional (R=-0,5). For the "average degree of openness" of 60% of all observed flowers (R=0,3-0,5) and for the "average degree of submergence" of 20% flowers (R=-0,4... -0,5) in this photoperiod the similar dependence was seen. The flower "average degree of submergence" was also directly proportional (R=0,4-0,5) to the top layer water temperature and air temperature. A similar dependence was noted for 60% of flowers which were observed in given photoperiod.
Visual analysis of "average degree of flower openness" and "average degree of submergence” points dependence on "absolute time" (fig. 2) allowed us to observe the following features of these flowers’ behavior.
Figure 2. The change of "average degree of flower openness" and "average degree of flower submergence" for N. candida during natural photoperiod in the Acrtic Circle region.
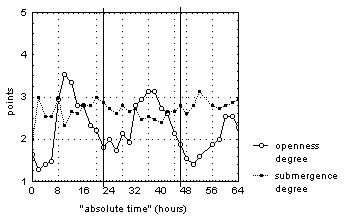
Vertical continuous lines mark 00:00 time for each day of observations.
The behavior of more then half of the flowers was different from that described in the literature. Also, we saw short-term (not more then an hour) opening flowers of floating on the water surface, momentary submergence of half-opened and fully opened flowers, and long-term (about 12 hours) continuation in a half-sunken condition of opened flowers.
The behavior described above was observed for flowers at different development stages and in different times of day, which is why we can say about the endogenous nature of these deviations from the literature’s description of flower behavior. Less then half of the observed flowers opened daily for the time from 2 to 12 o’clock, while in nearly all cases open flowers were not submerged into water. The rest of the flowers opened in one or two out of three days of research, and they were not submerged into water. All flowers opened at different times of day and for different length of the time, which did not depend on the development stage or on weather conditions. It is noteworthy that most of the observed flowers spent most of the time on the water surface or halfly submerged under it. At the same time, the flowers were open for only about one fourth of the observation time (table 3).
Table 3. Some characteristics of flower behavior during different photo rates, when being not on bud stage, not on terminal stage.
| Characteristic | In mid region Natural photo rate | in Polar circle region Natural photo rate | Darkness | Flash light during dark period of the day | |
| Averaged part of time when flower was…% from all the observation time | In submerged state | 15 | 30 | 30 | 5 |
| On the water surface | 30 | 46 | 20 | 68 | |
| In open state | 20 | 47 | 5 | 25 | |
| In closed state | 50 | 30 | 23 | 47 |
From correlation analysis it is known that only 40% of observed flowers had a negative correlation (R=-0,5... -0,8) between the "average degree of flower openness" and "average degree of submergence". "Average degree of openness" correlates (R=0,4-0,9) with each of the observed flowers’ degree of flower openness in the given photoperiod. "Average degree of submergence" correlates (R=0,4-0,6) with degree of submergence in only half the flowers. Thus, "average degree of flower openness" identically characterizes the change in degree of openness for each of the observed flowers in the given photoperiod, while "average degree of submergence" describes only the common tendencies of change in degree of submergence for each of the flowers.
Visual analysis of the cause-and-effect diagram for "average degree of openness" and "average degree of submergence” makes it possible to find the general tendencies of flower behavior. Generally, flowers appear to be on the water surface. Every day they are usually open from 8:00 until 12:00-14:00. The "average degree of flower openness" changes over a 24-hour period and the "average degree of flower submergence" changes aperiodically. However, as we can see from each of the flowers’ behavior descriptions, there are exeptions to every common rule. However, different flowers’ behavior correlates slightly with that of the others.
All flowers’ "average degree of openness" during the given photoperiod are positively correlated (R=0,5-0,7) with upper water level temperature and air temperature.
"Average degree of openness" and "average degree of submergence" correlate with degree of openness (R=0,5-0,8) and degree of submergence (R=0,5-0,6) for only a half of all flowers which were kept in darkness. That is why the average value describes only the general tendencies of flower behavior in this photoperiod.
During the observation period, regardless of the daytime and "absolute time" flowers were floating on the water surface, sometimes half submerged under it (fig. 3).
Figure 3. The change of "average degree of flower openness" and "average degree of flower submergence" for N. candida during the experiment without the light period.
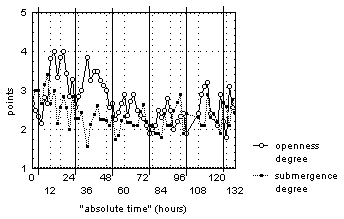
Vertical continuous lines mark 00:00 time for each day of observations
During the first two days of our observations, flowers opened in the daytime from 8:00 until 14:00-18:00, and they stayed half-open at night. Flowers were always half open (fig. 3) for the next 3 days of our observations, which may have been caused by the end of the blooming period of most of observed plants. This is typical for 70% of all flowers. The "average degree of flower openness" changed over an approximately 24-hour period, in contrast to the arhythmic changes in "average degree of flower submergence".
Behavior analysis for individual flowers demonstrates much smaller synchronic change in openness degree (flowers are open from 4:00-12:00 until 14:00-24:00). A small flow of water into the flower when half-open flowers were submerged under the water appeared for 70% of observed flowers. This description demonstrates the lack of negative dependence typical of the natural photoperiod between degree of flower openness and degree of submergence. This was confirmed by data correlation analysis.
No clear connection between flower behavior and registered weather conditions was found.
"Average degree of openness" and "average degree of submergence" correlate with degree of openness (R=0,5-0,9) and degree of submergence (R=0,6-0,8) for all flowers, which were subjected to a flash of light at night. Thus, the averaged exponent can identically describe the behavior of all flowers, which were kept in this photoperiod. On the day after the flash, the flowers were open from 10:00 until 16:00, and were also fully submerged under the water. During the first two nights (from 0:00 until 4:00-8:00) after lightening flowers were closed and submerged under the water. At all other times the flowers were half-open and half- or fully submerged under the water. This did not depend on flowers’ development stage, time or registered weather conditions (fig. 4).
Figure 4. The change of "average degree of flower openness" and "average degree of flower submergence" for N. candida during the experiment with the flash during the night.
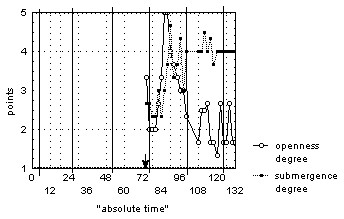
Vertical continuous lines mark 00:00 time for each day of observations. Arrow points at the moment of the flash.
A few flowers were found open under water or floating closed on the surface. The data are insufficient to calculate the period of changes in "average degree of flower openness". However, our data do not contradict the hypothesis of a 24-hour period in this case also. This description demonstrates the absence of inverse correlation typical for the natural photoperiod between degree of flower openness and degree of flower submergence; this also can be confirmed by correlation data analysis.
Flower behavior during the second period of observations could have been caused by the end of their blooming. It is significant that most time flowers were half-open and floating on the water surface and/or open for a very short time (table 3).
Correlation data analysis shows that the "average degree of openness" of these flowers is directly proportional to PSR intensity (R=0,8) and on upper level water temperature (R=0,5).
Certain common tendencies in the change in degree of flower openness could be seen during different photoperiods and not only during the natural photoperiod in the mid-Russia region. Changes in flowers’ degree of openness resemble circadian cycles for all photoperiods. This suggests that the change in degree of openness is a rhythmic endogenous process (Ehmme, 1965).
The degree of flower submergence changes with almost no observable periodicity. This fact is usually expressed less then nonlinear trend. In the mid-Russia region, negative dependence is seen during natural photoperiod between the degree of openness and degree of submergence point. From this, it follows that for the change in the degree of submergence weak rhythmic of exogenous nature reside (Ehmme, 1965).
The change in the degree of flower openness may be observed Nymphaeaceae family flowers for constantly floating on the water surface (Sculthorpe, 1967). The degree of flower openness determines a flower’s ability to pollinate, that is its ability to carry out main reproductive function. This may explain the presence of endogenous rhythms if change in the degree of flower openness as distinct from the degree of submergence.
It is appears that the change in degree of flower openness is explained by perianth cells activity, while the degree of flower submergence is controlled by peduncle cells. Many authors have repeatedly established the loss of reciprocal synchronization for plant cells, as well as the main role of light in their synchronization (Wilkins, 1964; Ehmme, 1965; Winfri, 1990 and others). Moreover, the degree of flower openness and degree of flower submergence may change independently of each other, and they may be synchronized with the change of light intensity. This conclusion is at variance with V.A. Stein-Margolina's opinion (1982) considering the possibility of mutual synchronization of perianth and peduncle cells. The usual connection between the degree of flower openness and the degree of it's submergence disappears in case of disparity between the given photoperiod and the natural mid-Russia region photoperiod.
Next time we plan to observe water-lilies in experiments with different lengths of day light and with reduced intensity of sunlight in Tverskaya district in order to determine the role of light in the behaviour of water-lily flowers more conclusively.
The positive correlation between degree of flower openness, top layer water temperature and air temperature which was seen during this and previous years (Volkova et al., 2002) may have been caused by daily variation these factors. The lack of such a connection in the experiment without a light period demonstrates this is the case. No correlation between flower behavior and the average air humidity was found, which confirms with our earlier results (Volkova et al., 2002) and contradicts those of A. Kerner fon Marylaun (1891). Likewise no influence of atmospheric pressure on rhythmic changes in openness degree and flower submergence degree has been discovered. This suggests that the only reason for flowers to close periodically is increase in flower insect pollination effectiveness (Kerner fon Marylaun, 1891; Wiersema, 1988).
The studies took place in the Moldino Biological station of Moscow South-West High School, and also during a high school expedition to the White Sea coast under the guidance of Dr. S.M. Glagolev. Dr. A.B. Shipunov, Prof. V.N. Tutubalin, Dr. D.D. Sokolov and Prof. I.A. Shulgin made valuable comments on earlier drafts. School teacher A.N. Kvashenko, post-graduate student of the MSU Biology faculty J.V. Kosenko, and high school students took an active part in the fieldwork led by Dr. A.B. Shipunov. MSU meteorological observatory senior research assistant Dr. O.A Shilovceva allowed us to use the meteorological equipment.
Artamonov, V.I., 1989. Green forcasters. Moscow. [in Russian]
Bunnig, E., 1931. Untersuchungen uber die automen tagesperiodischen Bewedungen der Primarblatter von Phaesolus multiforus. Jahrb. Wiss. Bot., 75, 439-480.
Downey, E.C., 2002. Xephem. Astronomy program. Version 3.2.2. [Electronic resourse]. Mode of access: http://www.clearskyinstitute.com/xephem/.
Ehmme, A.M., 1965. The clock of living nature. M., Peace.
Kerner fon Marylaun, A., 1891. Pflanzenleben. Bd. 2. Leipzig, Bibliographisehes Inst.
Kleshnin, A. F., 1954. Plant and light. Moscow. [in Russian]
Mahotkina, E.L., 1983. Colour piranometres. Vol. 456. Moscow. [in Russian]
Sculthorpe, C.D. 1967. The biology of aquatic vascular plants. London.
Suini, B., Gastings, J., 1964. The influence of the temperature on the circadian rithmes. In: Biological clocks. Moscow. [in Russian]
StatSoft, Inc., 1999. STATISTICA for Windows [Computer Program manual]. Tulsa, OK.
Stejn-Margolina, V.A., 1982. Some aspects of leaves movement. Progress of modern biology, 94, N 1(4), 111-125. [in Russian]
Velde, G., 1986. Developmental stages in the floral biology s. l. of dutch Nymphaeacaea (Nymphaea alba L., Nymphaea candida Presl., Numphar lutea (L.) Sm.). Acta Bot. Neerl., 35, N 2, 11-113.
Volkova, P.A, Sonina, S.I., Shipunov, A.B., 2002. The perticularities of white water-lily flowers behaviour (Nymphaea candida Presl.) in the Moldino lake (Tverskaya province). Bulletin MOIP, biol. dep. 105, N 5, 57-63. [in Russian]
Voskresenskaja, N.P., 1965. Photosintesis and spectral composition of the light. Ì., 6, 161, 210-215, 256. [in Russian]
Wiersema, H.J., 1988. Reproductive biology of Nymphaea (Nymphaeacaea). Annals of the Missouri Botanical Garden, 75, N 3, 796-804.
Wilkins, M., 1964. The temperature influence on the plants rhythms In: Biological clocks. Moscow. [in Russian]
Winfri, A.T., 1990. Time on biological clocks. Moscow. [in Russian]