Detailed investigation of the morphological variability of Nymphaea species was developed on 48 populations in the European Russia. We studied both macromorphological characters in field (including analysis of leaf shape with the geometrical morphometry) and palynology. Only one polymorphic species, N. candida, growths in the most part of European Russia. Nymphaea tetragona is absent on the investigated territory, and N. alba have been found only in Astrakhan region (Volga river mouth). These three species are separated by several morphological characters of fresh plants. Nymphaea tetragona differs from N. alba and N. candida on the sculpture of exine of proximal part of pollen grains. Size characters of Nymphaea leaves and flowers do not depend on organic content in water.
Nymphaea species have high morphological plasticity. Size of leaves and flowers and also some qualitative characters of flowers are thought as strongly depended on hydrological (especially temperature) and edaphic conditions (Heslop-Harrison 1955, Kupriyanova 1976, Dubyna 1982). However, quantitative estimation of edaphic conditions was not performed in the abovementioned studies. Size of different parts of Nymphaea species also depends on age of the plant (Gluck 1924 as cited in Muntendam et al. 1996, Dubyna 1982).
The most common opinion is that there are three Nymphaea species in Russia: N. alba L., N. candida Presl. and N. tetragona Georgi. Nymphaea alba is spreaded across all European Russia, N. candida growths both in the European Russia and Siberia, and N. tetragona occurs in Siberia, in the Russian Far East and in the Kola peninsula (Komarov 1937; Muntendam et al. 1996). Similar values of qualitative diagnostic characters are pointed out in all analyzed sources, so in theory, these characters are able to distinguish these three species (Table 1). Unfortunately, many main diagnostic characters (e.g. color and shape of the stigma, shape of cup base etc.) change or disappear even after very careful pressing in herbaria (Lisitsyna 2003). Moreover, the differences in the leaf blade shape, which appears to be a frequently used diagnostic character (Komarov 1937, Lisitsyna et al. 1993, Uotila 2000), before and after herbarization are very similar with interspecific differences, i.e. leaves with leaf blade shape which is typical for N. alba obtain more typical for N. tetragona shape after the herbarization (Volkova 2007). That is why it is so important to investigate morphology of white water-lilies on the fresh material (Uotila 2000).
Table 1. Main diagnostic characters for distinguishing Nymphaea species in Russia (data from literature)
| Character | N. alba | N. candida | N. tetragona |
| Shape of cup base | round | rounded-quadrangular | quadrangular with prominent ribs |
| Shape of filaments of inner stamens | linear | lanceolate | oval |
| Sculpture of pollen grains exine | baculums | verrucas | granular |
| Number of stigma lobes | (7) 8-20 (23) | 6-14 (20) | (4) 5-10 (16) |
| Shape of stigma disc | almost flat (slightly concave) | strongly concave | strongly concave |
| Color of stigma disc | yellow | yellow, orange, red | yellow, red, purple |
| Shape of the central stigma projection | short spherical | long conical | long conical |
| Flower diameter (cm) | (3) 5-15 (20) | (3) 5-11 (16) | 3 (and less) –6 (10) |
| Ovary appearance | does not become narrower near stigma, covered up to the top with scars of fallen stamens | become narrower near stigma, is not covered up to the top with scars of fallen stamens | become narrower near stigma, is not covered up to the top with scars of fallen stamens |
| Bud shape | oblong-ovoid with obtuse top | oblong-ovoid with acute top | four-sided pyramid |
| Leaf shape | widely-elliptic either rounded-ovate or rounded widely-elliptic | either rounded-ovate or rounded elliptic | either rounded-ovate or rounded |
| Shape of main leaf veins | almost straight | bent along the full length | bent only in the first third of the length |
| Length of the leaf (cm) | (10) 15-30 (35) | (6) 12-26 (30) | (4) 5-9 (20) |
| Width of the leaf (cm) | (8) 14-27 (35) | (8) 12-24 (30) | (3) 4-10 (16) |
However, there is the opinion that N. candida and N. alba can be distinguished with certainty only basing on size, shape and exine sculpture of pollen grains (Kupriyanova 1976, Muntendam et al. 1996, Uotila 2000). L.A. Kupriyanova (1976) showed that Nymphaea pollen grains in the European part of the USSR are characterized by high morphological stability and can be used for distinguishing species; this opinion based mainly on the exine sculpture. Interspecific hybrids of Nymphaea are characterized by lower fertility (Komarov 1937, Heslop-Harrison 1955) and various morphology of pollen grains (Kupriyanova 1976). One should note that these investigations were conducted on the small samples (1-3 flowers per species), while there is large variance of the palinomorphology even within one Nymphaea population (Volkova 2007), which let us consider these studies as preliminary ones. Investigations of J.B. Muntendam with colleagues (1996) were carried out on the SEM-micrographs; this method does not let estimate correctly the shape and the sizes of intact pollen grains because of their deformation during scanning microscopy in the high vacuum (Volkova 2007). This is almost all existing literature on diagnostic palinomorphology of European Nymphaea species, as far as we know, although pollen characters are mentioned in many wide-spread keys and proceedings as diagnostic ones (e.g. Komarov 1937, Dubyhna 1982, Tzvelev 2000, Uotila 2000). In contrast to the previous investigations, V.A. Poddubnaya-Arnoldy (1967) noticed that structure, size and shape of pollen grains can vary significantly within one species, although these are diagnostic characters.
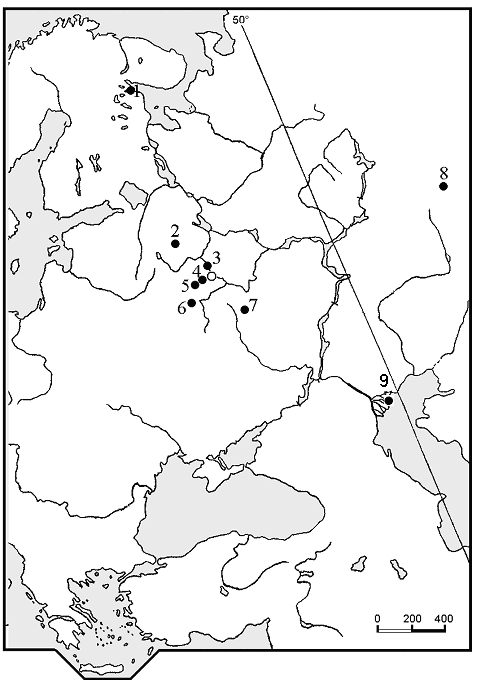
We investigated 48 populations of white water-lily from 47 reservoirs and rivers in Karelia republic, Moscow, Tver, Kaluga, Chelyabinsk, Lipetsk and Astrakhan regions of European Russia (Fig. 1). We also investigated three populations of N. tetragona on the shore of lake Bajkal (Western Siberia), situated close to the type location of this species (Krupkina 2001), and two populations of N. alba s.l. from there for comparison with populations from European Russia. Data were collected from June to September in 2003-2005.
Figure 1. Collection sites for the investigated populations. Population numbers refer to the Table 2. Investigated populations in Western Siberia (shore of the lake Bajkal) are not shown. 1—Karelia republic (populations number 120, 130-138, 206-211); 2—Tver region (101-116); 3—Moscow region (117, 118); 4—Moscow region (119); 5—Kaluga region (128, 129); 6—Lipetsk region (127); 7—Chelyabinsk region (121); 8—Astrakhan region (213-217).

Table 2. Investigated populations of Nymphaea spp.
| population number | name of stream or reservoir | saprobity index1 | proportion of fertile pollen grains (%)2 | class of water purity1 | type of the exine sculpture3 |
| 14. Karelia republic, Loukhi region | |||||
| 120 | Lake Indigo | 0.68 | … | 2 | 4 |
| 130 | Lake Tikhoe | 0.8 | 80 | 2 | 1 |
| 131 | Lake Gamarbiya | 0.75 | … | 2 | 1 |
| 132 | Peatbog near Lake Gremyakha | … | 89 | … | 2 |
| 133 | Lake Sennoe | 0.85 | … | 2 | 2 |
| 134 | Lake Evrika | 0.7 | 100 | 2 | 2 |
| 135 | Lake Speloe | 0.7 | 93 | 2 | 4 |
| 136 | Lake Kh | 0.65 | 38 | 2 | 2 |
| 137 | Lake I | 0.75 | 98; 88 | 2 | 2 |
| 138 | Lake La | 0.78 | 94 | 2 | 1 |
| 206 | Riv. Sinyaya | … | 97 | … | 2 |
| 207 | Lake Verkhnyaya Pazhma, western part | … | 98; 93 | … | 2 |
| 208 | Lake Verkhnyaya Pazhma, eastern part | … | 82; 88; 99 | … | 4 |
| 209 | Mouth of riv. Nol'ozyorskaya | … | 99 | … | 2 |
| 210 | Head of riv. Nol'ozyorskaya | … | … | … | … |
| 211 | Lake Tajnoe | … | 98 | … | 1 |
| 2. Tver region, Udomlya environs | |||||
| 101 | Lake Zaverkhov'e | 1.7 | 100 | 3 | 4 |
| 102 | Lake Matras | 1.4 | 100 | 2 | 2 |
| 103 | Lake Klin | 1.24 | … | 2 | … |
| 104 | Lake Borovno | 1.4 | … | 2 | 2 |
| 105 | Lake Belen'koe | 1.4 | 100 | 2 | 4 |
| 106 | Lake Golovets | 1.87 | 95 | 3 | 3 |
| 107 | Lake Perkhovo | 1.4 | … | 2 | 2 |
| 108 | Lake Moldino, eastern part | 1.6 | … | 3 | 1 |
| 109 | Lake Moldino, north-eastern part | 1.6 | … | 3 | 4 |
| 110 | Lake Moldino, central part | … | 99 | … | 2 |
| 111 | Lake Rogozno | … | 33 | … | 4 |
| 112 | Lake Turishino | … | 99 | … | 2 |
| 113 | Lake Soroka | 2 | 5 | 3 | 4 |
| 114 | Lake Volkovo | 1.5 | 97 | 3 | 4 |
| 115 | Lake Pisoshno | 1.4 | 95 | 2 | 4 |
| 116 | Lake Pochaevo | 1.5 | 63 | 3 | 2 |
| 3-4. Moscow region | |||||
| 117 | Pond Sterlyazhij near Zvenigorod | 1.8 | 77; 87 | 3 | 4 |
| 118 | Lake Sima near Zvenigorod | 0.95 | … | 2 | 2 |
| 119 | Pond near tourists base near Mozhajsk | 1.4 | 95 | 2 | 2 and 3 |
| 5. Kaluga region, Mosal'sk district | |||||
| 128 | Riv. Ressa, upper stream | 1.5 | 100 | 3 | 1 |
| 129 | Riv. Ressa, lower stream | 1.7 | 100 | 3 | 1 |
| 6. Lipetsk region | |||||
| 127 | Lake Mokhovoe, near Lipetsk | … | 91 | … | 2 |
| 7. Chelyabinsk region, Bredy district | |||||
| 121 | Riv. Karaganka | 1.9 | 93 | 3 | 2 |
| 8. Astrakhan region, delta of the Volga river | |||||
| 213 | Erik Finogenov | … | … | … | … |
| 214 | Erik Volodarovskij | … | 98 | … | 1 |
| 215 | Kultuk Pryamoj Lotosnyj, northern part | … | 95 | … | 1 |
| 216 | Kultuk Pryamoj Lotosnyj, central part | … | 97 | … | 1 |
| 217 | Krep' Blinovskaya | … | 98; 100 | … | 1 |
| 9. Western Siberia, near the Lake Bajkal | |||||
| 201 | Lake near road Irkutsk—Ulan-Ude, 905 km | … | … | … | 3 |
| 202 | Ponds of Bajkal pulp and paper plant | … | 80 | … | 2 |
| 203 | Lake near road Irkutsk—Ulan-Ude, 202,5 km | … | … | … | 3 |
| 204 | Peatbog Leshkovskoe | … | 96 | … | 3 |
| 205 | Ponds near railway station Slyudyanka | … | 93 | … | 2 |
1 -- according to Sladechek, 1967
2 -- data for several flowers from the same population are separated with
semicolon
3 -- proximal part of the pollen grain was investigated, for exine types
description see Results
4 -- number of region corresponds to the Fig. 1
We tried to investigate not less then 15 plants per population; however, there were populations with fewer plants, so 344 plants were investigated in total. Six qualitative and six quantitative characters (which are mentioned as diagnostic in most keys and proceedings) were observed for each plant (Table 1, Fig. 2). The amount of organic in the water were estimated via saprobity index, which was determined from list of indicator species of diatoms and their abundance in the water sample (Sladechek 1967).
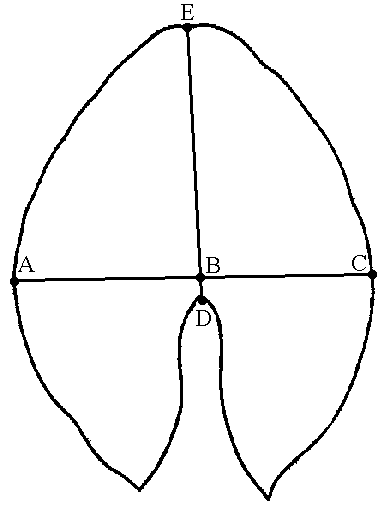
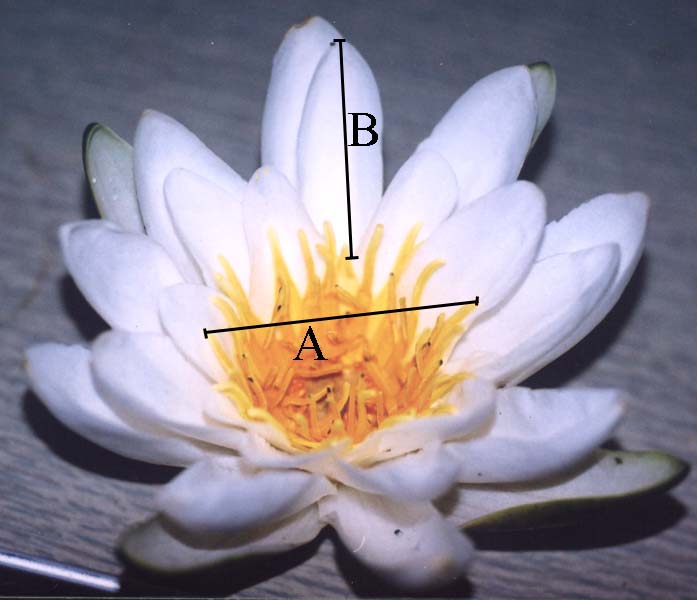
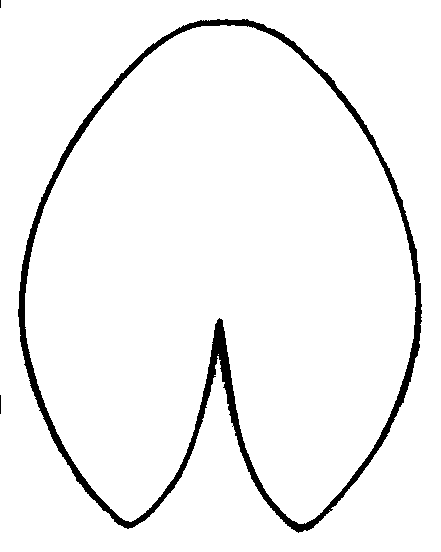
Figure 2. Investigated quantitative characters of Nymphaea leaves and flowers
| A — Leaf: AC—width, DE—length, BD—position of the maximum width. | B — Flower: A—diameter of the circle of outer stamens, B—length of the outer petal |
 |
 |
To digitize contours, we used 100 equally spaced landmarks (first landmark located on the base of leaf). This algorithm of producing (pseudo)landmarks let us compare objects shapes without getting any information about biological sense of observed differences (Kores et al. 1993), which entirely fits our aim. The coordinates of the landmarks were written in the data file with help of screen digitizer tpsDig (Rohlf 2006). The coordinates of consensus configuration and also the values of main, relative and partial warps, characterizing the degree of differences between the specimen and consensus configuration were calculated with help of program tpsRelw (Rohlf 2007), which realizes the idea of geometrical morphometry in prototype of primary component analysis. Original coordinates were normalized by Procrustes fit method (alpha = 0).
We investigated the dependence of leaf shape on its position on the rhizome with help of tpsRegr (Rohlf 2005) software; the fit to the regression model was tested using a generalization of Goodall’s (1991) F-test. Averaging of leaves blade shape was done with help of program tpsSuper (Rohlf 2003). Data files were edited and converted with help of auxiliary program tpsUtil (Rohlf 2000).
Pollen fertility was assessed on herbarized flowers by the acetocarmin staining method (Radford et al. 1974) on 100 pollen grains per flower. All unstained or faintly stained pollen grains were considered sterile (Table 2).
We used multivariate analysis of variance, nonparametric correlation analysis, nonparametric Wilcoxon test and parametric Student test for detection differences and connections between variables. Shapiro-Wilks test was performed to test normality. Kruskal non-metric multidimensional scaling (hereafter MDS: Ripley 1996) of similarity matrices computed with “daisy” (Kaufman & Rousseeuw 1990) metrics was used for the classification based on the morphology; last method was specially developed for data with mixed type of variables (both continuous and discrete) such as our data. Principal component analysis (hereafter PCA) was used for classification of the leaves shapes (because in this case we deal with continuous variables of relative warps matrix, Pavlinov 2001). Dichotomous recursive classifications (Ripley 1996) were used for calculations of the typical character values during the preparation of the diagnostic key. All calculations and graphs creation were made in R environment for statistical computing (R Development Core Team 2005).
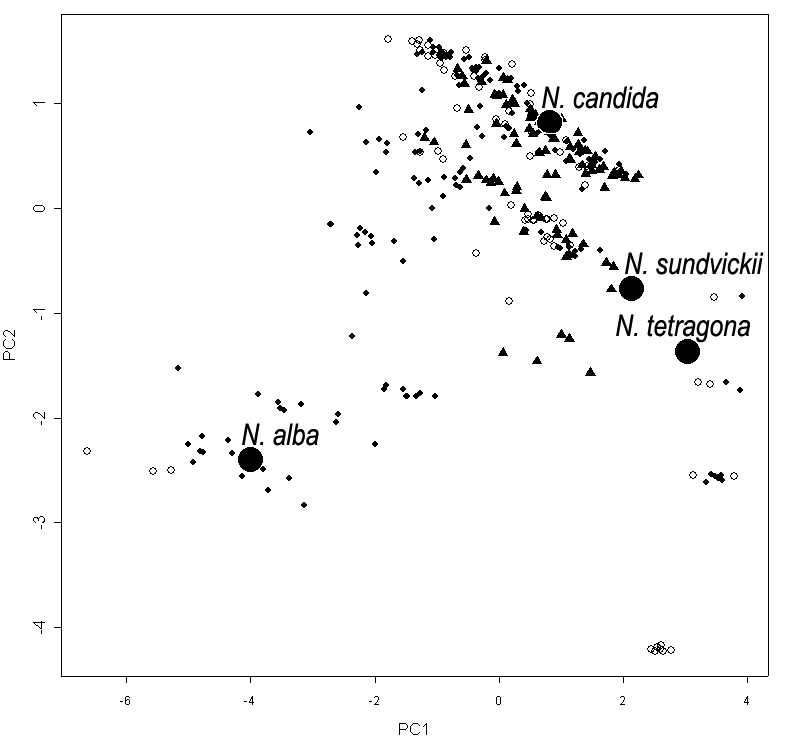
Figure 3. Multidimensional scaling of morphometric data for Nymphaea in European Russia. “Anchor specimens” are marked with black circles.
A. Each plant is marked by the number of the region (see Fig. 1, Table 2).
B. Each plant is marked by the symbol, according to the size of pollen
grains:
filled circles—“small” pollen, filled triangles—“large pollen”, open
circles—unknown pollen size.
Values of morphological characters which were typical for each of distinguished groups were calculated and used for the diagnostic key of three European Russian Nymphaea species. We used such simple and reliable characters as shape of the flower base, shape of filaments of inner stamens, number of stigma lobes, size of the leaf and flower size. This key is appropriate for distinguishing Nymphaea species in nature but not in the herbarium, which can be considered as weakness of the key, caused, however, by the peculiarities of our object.
2. Cup base is rounded (view from peduncle). Filaments of inner stamens are
linear. Leafs cover each other, raising themselves over the water. Number of
stigma lobes exceeds 13. Length of the leaf exceeds 15 cm ..... N. alba L.
--- Cup base is rounded-quadrangulate, clear edges are never seen. Filaments of
inner stamens are linear or lanceolate. Leafs are floating on the water. Number
of stigma lobes is less then 13 cm, sometimes more but in this case length of
the leaf does not exceed 15 cm .....
N. candida Presl
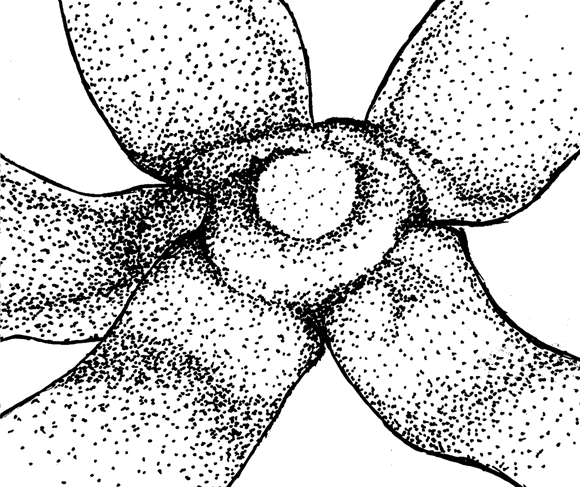
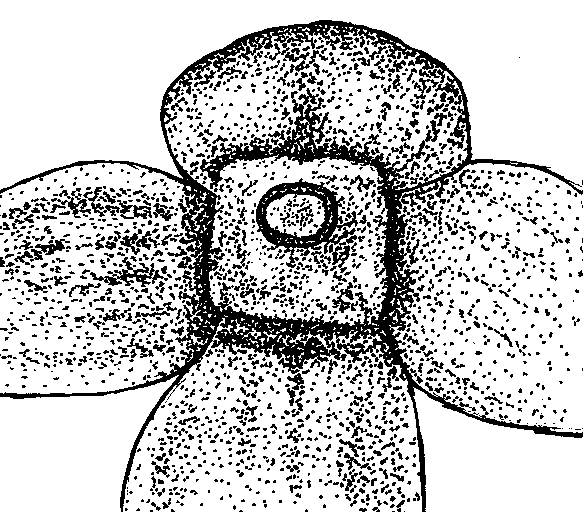
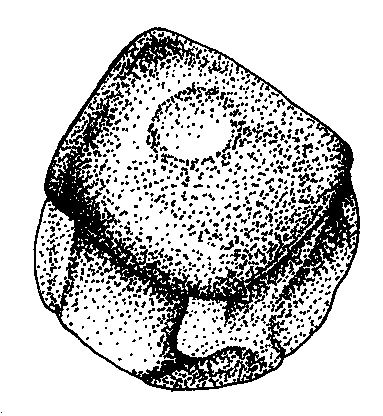
Figure 4. Qualitative characters, used for separation of three Nymphaea species in European Russia.
A-C — shape of flowers base (view from peduncle, not in one scale):
|
A.Nymphaea alba |
B. Nymphaea candida |
C.Nymphaea tetragona |
 |
 |
 |
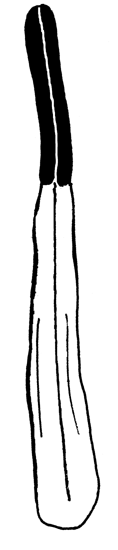
D-F — shape of the filament of inner stamen (in one scale):
|
D. Nymphaea alba |
E. Nymphaea candida |
F. Nymphaea tetragona |
 |
|
|
Figure 5. Primary component analysis of relative warps matrix (geometrical morphometry data) for leaf contours of Nymphaea in European Russia. Each plant is marked by the number of the region (see Fig. 1, Table 2). "Anchor specimens" are marked with black circles. Plants from Astrakhan region ("A zone") and from Siberia ("B zone") are separated.
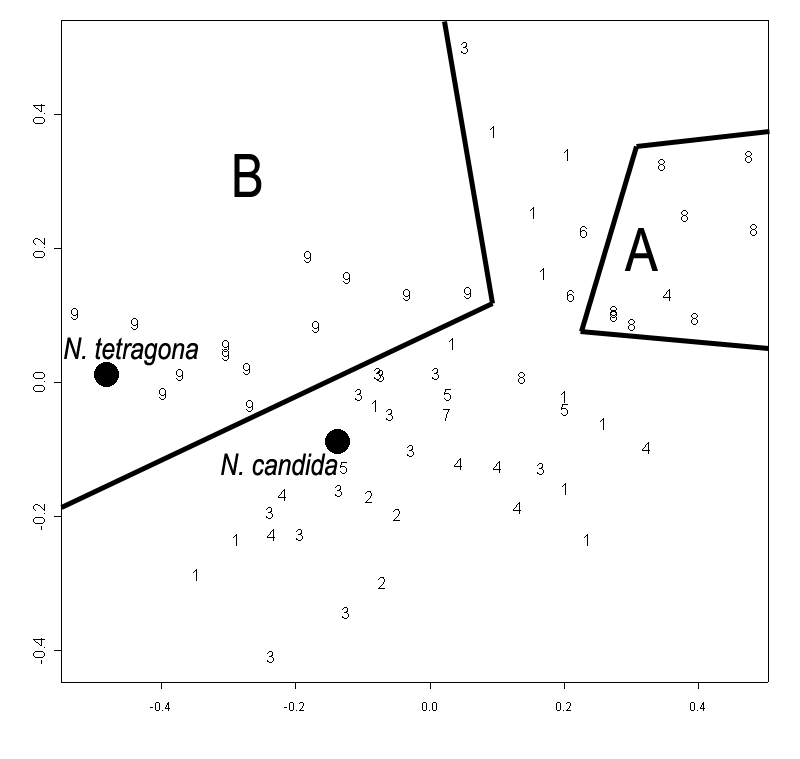
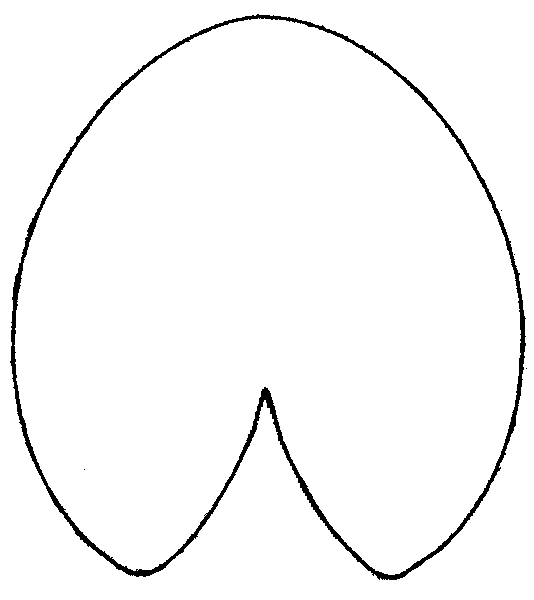
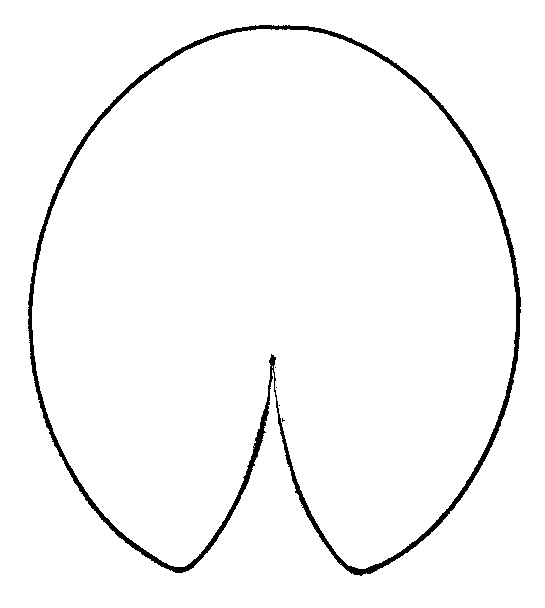
Figure 6. Averaged contours of leafs for three groups of leaves with different shape.
A — plants from Astrakhan region (Fig. 5, Zone A),
B — group of N. candida “anchor” (Fig. 5, central region),
C — plants from Siberia (Fig. 5, "B zone").
We discovered significant dependence (generalized Goodall F-test: p<0.05) of leaf shape on the leaf position on the rhizome (distance from the apex) for 6 of 11 investigated plants. However, the character of this dependence was not same for different plants. Leaves of two plants rounded and their lobes became more divergent, while moving in the acropetal direction, whereas leaves of two plants elongated and their lobes became less divergent, and leaves of the other two plants did not change their shape.
Plants of N. alba and N. tetragona morphotypes have “small” pollen. Plants with N. candida morphotype have both “small” and “large” pollen (Fig. 3b); it is not possible to distinguish separate morphotypes in N. candida according to the pollen size.
Pollen fertility in different plants from one population did not vary more then for 15%. Plants from vast majority of the populations have highly fertile pollen (more then 75% of fertile pollen grains), whereas one population (116) from Tver region have deferred pollen fertility (63%), two populations from Karelia (136) and from Tver region (111) have low pollen fertility (33-38%) and one population from Tver region (113) has almost sterile pollen (5% of fertile pollen grains), see Table 2. Pollen grains from plants of this last population were very small in number (10-20 pollen grains per anther) and deformed. Macromorphology of plants from this population was also quite original and combines characters of N. alba (linear filaments of inner stamens, yellow and flat stigma) and N. candida (conic central projection of stigma).
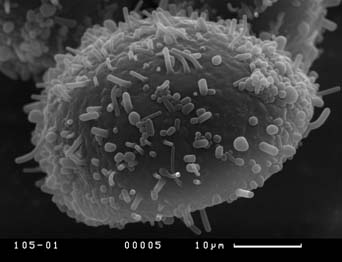
Visual analysis of SEM-micrographs revealed that exine sculpture is not homogenous on the whole surface of pollen grain. Sculpture of distal part of pollen grain does not demonstrate any discrete patterns, being consisted of different in size verrucas that become larger near equator. The exine sculpture of the proximal part of pollen grains is very diverse. Hereafter we will describe the exine sculpture of proximal part of pollen grain as “exine sculpture” since this part is the most perspective for distinguishing different pollen morphotypes in Nymphaea. Four main types of exine sculpture can be distinguished with some transitions between them:
Figure 7. Scanning electron micrographs of Nymphaea pollen grains, view from the proximal pole. Different types of the exine are shown. Population numbers refer to Table 2.
A — exine sculpture of the first type (population number 128);
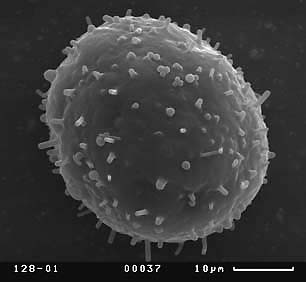
B — exine sculpture of the second type, variant with long baculums (population number 137);

C — exine sculpture of the third type, variant with prominent verrucas (population number 106);

D — exine sculpture of the fourth type (population number 105)
Exine sculpture frequently varies essentially within one population. The population from one pond in the Moscow region (population 119) seems especially interesting to us. Near the southern shore of the pond there are plants with N. candida morphotype (Table 1) and exine sculpture of the second type. In the middle of the pond there are plants with some morphological characters of N. tetragona (bent along the full length main veins of the leaf, strongly invaginated stigma—see Table 1) and exine sculpture of the third type. Three investigated populations in the large lake Moldino in Tver region (108-110) have various exine sculpture (Table 2) and very similar macromorphology (Fig. 3). We found the third type of exine sculpture in all herbarium specimens of N. tetragona and in all populations of N. tetragona collected by us near lake Bajkal (201, 203 and 204). This type of exine sculpture was also found in one lake in Tver region (population 106: Table 2). Plants in this population differed from N. candida morphotype by linear filaments of inner stamens, main veins which are bent along the full length of the leaf and strongly invaginated stigma (Table 1). All other populations have pollen of the first, second and forth types of exine sculpture without any dependence on plant macromorphology or region of growing (Table 2).
We found plants with typical for N. alba morphology only in the most southern part of European Russia (delta of the Volga river, Astrakhan region), plants with typical for N. tetragona macromorphology were found only in the Western Siberia. Our investigations do not support data about N. alba and N. tetragona in the middle Russia (Lisitsyna et al. 1993; Papchenkov 2003). According to our data, variety of Nymphaea morphotypes in the middle Russia can not be dissected into more or less discrete groups.
Separate marginal morphotypes of the morphological continuum, existing in middle Russia, can be interpreted as N. alba and N. tetragona. Specimens that have intermediate morphology between imaginary center of continuum (typical N. candida) and its marginal morphotypes can be also interpreted as hybrids N. alba × N. candida = N. × borealis and N. tetragona × N. candida = N. × sundvickii. This approach is driven to its logical end by V.G. Papchenkov (2003). However, this interpretation does not correspond with generally accepted species determination (Grant 1981). Distinguishing separate species on the base on small differences in morphology is not appropriate especially for plants with prevalence of vegetative reproduction over sexual (Elven et al. 2004), such us water-lilies (Heslop-Harrison 1955, Dubyna 1982).
We suppose that in the middle Russia there is only N. candida, a very polymorphic species. Our data about high morphological variability of N. candida agree with data of K.I. Aleksandrova (1996) for Lipetsk region of European Russia and contradict to data of D.V. Dubyna (1982) for the Ukraine.
Our point of view is supported by the information about existence of “hybrids” in the absence of parental species (Uotila 2000, Papchenkov 2003). As far as we know, these hybrids were distinguished only on the morphological basis so plants with unusual combinations of morphological characters lacking the hybrid original can be easily taken as “hybrids”. Our studies of pollen fertility show that plants with unusual combinations of morphological characters almost always have highly fertile pollen and therefore can be hardly consider as hybrids (Komarov 1937, Heslop-Harrison 1955, Neuhausl & Tomsovic 1957 as cited in Dubyna 1982).
Division of the investigated populations of N. candida on basis of the pollen size corresponds to their division on basis of macromorphological character set which probably means that two chromosomal races of N. candida exist in the middle Russia. This assumption agrees with data about the correlation between pollen size and ploidy level (Possubnaya-Arnol'di 1976) and about various chromosomal numbers known for the European N. candida (Heslop-Harisson 1955, Dubyna 1982, Wiersema 1988, Krupkina 2001). To test this hypothesis in future, it is essential to estimate ploidy level. However, these estimations will be troubled by big chromosomal numbers (up to more then 100) and small sizes of the chromosomes (Heslop-Harrison 1955). It is also essential to carry out DNA analysis for decision a question about species diversity in Nymphaea genus in the European Russia (Muntendam et al. 1996), what, as far as we know, was not done for the European Nymphaea species.