Flowers of Geranium sylvaticum L. are normally hermaphroditic and bear 10 stamens. Some flowers have anthers of all or several stamens reduced; these flowers belong to female or “intermediate” gender. This probably prevents the self-fertilization and lets female flowers produce more seeds or seeds with greater amount of nutrients, what favors to the population development (Asikainen, Mutikainen, 2003; Ramula, Mutikainen, 2003; Asikainen, Mutikainen, 2005). Negative connection was noticed between female flowers frequency and the seed production of hermaphroditic flowers in the populations (Asikainen, Mutikainen, 2003). Consequently, sex ratio in populations of G. sylvaticum deserves detailed investigations, being an important characteristic of reproductive status of plants in the population.
Frequency of female flowers was investigated in 23 Geranium sylvaticum populations in Finland (Asikainen, Mutikainen, 2003). The fraction of female flowers in the population varied from 0.4 to 27.2% (11.5% in average) and depended on habitat conditions. For example, female flowers are less frequent in favorable conditions, particularly in Southern Finland comparing to Northern Finland (Asikainen, Mutikainen, 2003). “Intermediate” flowers were found in the least half of investigated populations in very small amount (less then 1%). The only population from Southern Finland had 42% of “intermediate” flowers (Asikainen, Mutikainen, 2003).
G. sylvaticum is spread almost everywhere in Eurasia. That is why we decided to check data about influence of geographical latitude on the sex ratio in populations of G. sylvaticum on greater area of European Russia.
The investigations were carried out in the June–July 2003–2005 in northern part of Tver’ region (N 58°15', E 34°30'; 564 plants from 21 populations) and in northern part of Murmansk region on the Barents Sea shore (N 69° 10', E 36° 00'; 274 plants from four populations). During the research, we used the ecological definition of population (Waples, Gaggiotti, 2006). We counted number of fertile stamens for one flower from each flowering plant in the population. It was very important to investigate flower buds instead of open flowers because anthers easily drop from the stamen filaments on later stages of flower development what could cause an erroneous count of fertile stamens number. We calculated also some characteristics of habitat conditions (light intensity, moisture, soil acidity and degree of soil mineralization) via modified Ellenberg tables (Hill et al., 1999), using lists of most frequent vascular plant species in the locality.
The statistical analysis of our data was performed in R statistical environment (R Development Core Team, 2004). We used standard error of mean to show data variance around mean value.
Investigated regions have obvious differences in values of some edaphic factors calculated from the Ellenberg tables. We observe low soil mineralization in Murmansk region (2.0–3.0 points on the Ellenberg tables) comparing with medium or high mineralization in Tver’ region (3.5–7.5 points). Soil was moderately acid in Murmansk region (3.0–5.0 points) and weakly acid or neutral in the localities from Tver’ region (5.0–7.5 points).
We have not noticed any female flowers in all investigated populations in Tver’ region; and we have found the only one female flower in one of four investigated populations in Murmansk region (table 1). Thus, we found much greater rarity of female flowers then E. Asikainen and P. Mutikainen (2003).
Table 1. Frequency of plants of different sexes
|
region |
population number |
number of plants |
number of plants of given sex*: |
||
|
female |
“intermediate” |
hermaphroditic |
|||
|
Tver’ region |
1 |
6 |
0 (0) |
4 (66.7) |
2 (33.3) |
|
2 |
18 |
0 (0) |
15 (83.3) |
3 (16.7) |
|
|
3 |
20 |
0 (0) |
15 (75) |
5 (25) |
|
|
4 |
16 |
0 (0) |
11 (68.8) |
5 (31.2) |
|
|
5 |
18 |
0 (0) |
12 (66.7) |
6 (33.3) |
|
|
6 |
40 |
0 (0) |
15 (37.6) |
25 (62.5) |
|
|
111 |
17 |
0 (0) |
7 (41.2) |
10 (58.8) |
|
|
201 |
26 |
0 (0) |
5 (19.2) |
21 (80.8) |
|
|
202 |
36 |
0 (0) |
5 (13.9) |
31 (86.1) |
|
|
203 |
28 |
0 (0) |
4 (14.3) |
24 (85.7) |
|
|
204 |
59 |
0 (0) |
18 (30.5) |
41 (69.5) |
|
|
205 |
14 |
0 (0) |
3 (21.4) |
11 (78.6) |
|
|
206 |
31 |
0 (0) |
7 (22.6) |
24 (77.4) |
|
|
207 |
18 |
0 (0) |
6 (33.3) |
12 (66.7) |
|
|
208 |
9 |
0 (0) |
0 (0) |
9 (100) |
|
|
209 |
8 |
0 (0) |
1 (12.5) |
7 (87.5) |
|
|
210 |
61 |
0 (0) |
7 (11.5) |
54 (88.5) |
|
|
211 |
33 |
0 (0) |
0 (0) |
33 (100) |
|
|
212 |
28 |
0 (0) |
0 (0) |
28 (100) |
|
|
213 |
23 |
0 (0) |
2 (8.7) |
21 (91.3) |
|
|
214 |
61 |
0 (0) |
0 (0) |
61 (100) |
|
|
Murmansk region |
221 |
108 |
1 (0.9) |
3 (3.7) |
104 (96.3) |
|
222 |
108 |
0 (0) |
2 (1.9) |
106 (98.1) |
|
|
223 |
41 |
0 (0) |
0 (0) |
41 (100) |
|
|
224 |
18 |
0 (0) |
0 (0) |
18 (100) |
|
* number of plants of given sex (number of plants of given sex to total plant number in the population ratio, %)
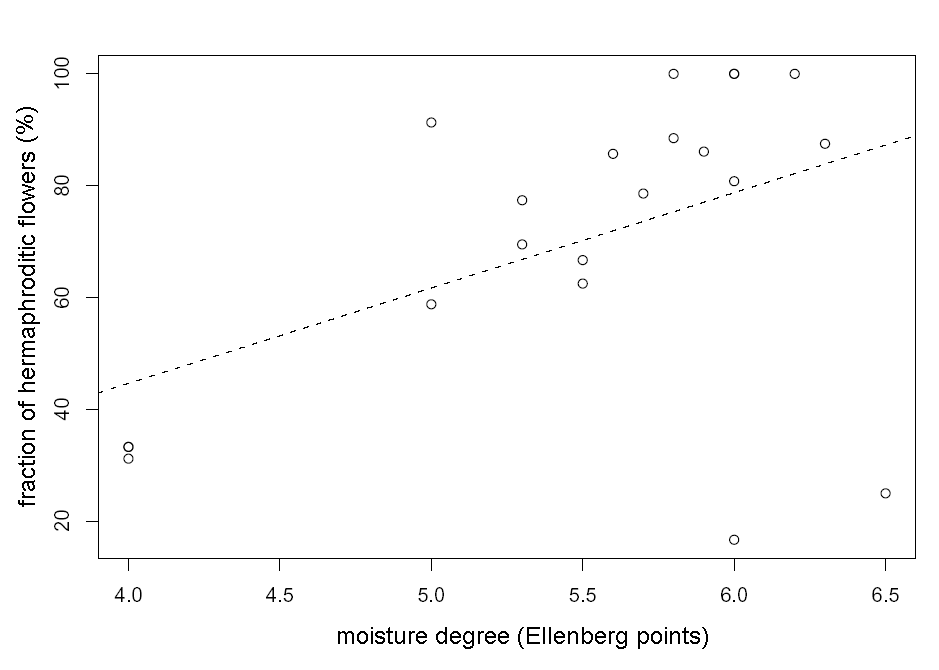
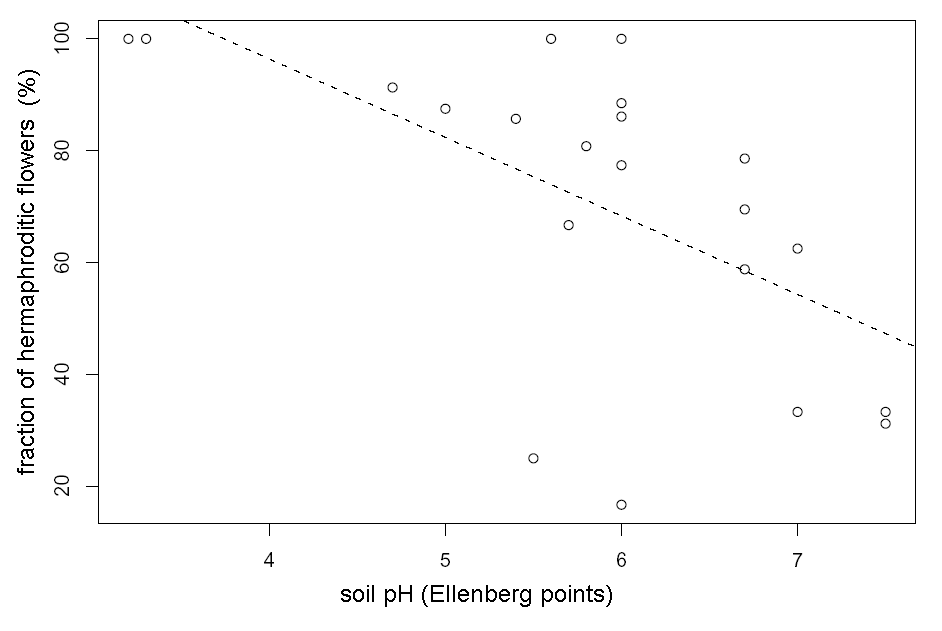
The fraction of hermaphroditic flowers in temperate Tver’ region is lower in average (57+/–6 %) then in Arctic Murmansk region (97+/–0.5 %), which is contrary to E. Asikainen and P. Mutikainen (2003) opinion (see also Table 1). The fraction of hermaphroditic flowers in Tver’ region depends on edaphic factors. We have found significant positive association between fraction of hermaphroditic flowers in the population and moisture degree (Multiple r-squared: 0.21, F-statistic: 5.18, p-value: 0.034, see also Fig. 1) and significant negative association between fraction of hermaphroditic flowers and soil pH (multiple r-squared: 0.35, F-statistic: 10.34, p-value: 0.005, see also Fig. 2).
Figure 1. Connection between fraction of hermaphroditic flowers in the population and moisture degree in Tver’ region. The line of linear regression (y=17x-23.5) is dashed.

Figure 2. Connection between fraction of hermaphroditic flowers in the population and soil pH in Tver’ region. The line of linear regression (y=-14x+152.5) is dashed.
Fraction of “intermediate” flowers in the population varies from 9 to 84% in Tver’ region (43+/-6% in average) and from 0 to 4% (1.5+/-1%) in Murmansk region (Table 1). Hereafter, our results confirm Finnish data (small frequency of “intermediate” flowers) only for Murmansk region.
Hereby, our data for sex ratio in different population of G. sylvaticum in European Russia correspond poorly with data of E. Asikainen and P. Mutikainen (2003) on Finnish populations of G. sylvaticum. These differences could be associated with different sizes of populations (investigated Russian populations are much smaller then Finnish), because it is known from other plants that variance in sex ratio increased with decrease of population size (Plantago maritima investigations by Nilsson and Agren, 2006). We can also suppose the existence of temporal (Agren, Willson, 1991) or longitudinal variation of frequency of female flowers in population of G. sylvaticum. Finally, the bias of the sex ratio in populations could be explained if the Finnish researchers have been examined open flowers instead of flower buds (some anthers can drop from the stamen filaments and full hermaphroditic flowers could be considered as “intermediate” and intermediate as “female”). Unfortunately, the flowering stage is not stated in their papers (Asikainen, Mutikainen, 2003; Ramula, Mutikainen, 2003; Asikainen, Mutikainen, 2005). However, Eija Asikainen (pers. comm.) said that in their investigations all stamens were accurately counted several times during flowering. So, we need to reject this explanation.
Variation of frequency of female flowers in populations of G. sylvaticum seems to have more complex patterns, then it was thought before (Asikainen, Mutikainen, 2003; Ramula, Mutikainen, 2003; Asikainen, Mutikainen, 2005). The frequency of female plants in gynodioecious populations may vary widely over relatively short distances (Couvet et al., 1990, cited after Williams et al., 2000). Some stochastic processes could have influence to the sex ratio in small gynodioecious populations (Nilsson, Agren, 2005). We hope that further investigations throughout all the area of G. sylvaticum with attention to stages of flowers development will provide sufficient information on sex in G. sylvaticum populations.
All fieldwork was made during summer practices of Moscow South-West High School lead by Dr. S. Glagolev. We thank O. Aleksandrova, N. Guryanova, E. Kost, O. Kostereva, O. Kudina, K. Markvichyova, D. Mordvinkin, E. Peskova and P. Tsesarskij for their essential help in the field.
Asikainen E., Mutikainen P. (2003) Female frequency and relative fitness of females and hermaphrodites in gynodioecious Geranium sylvaticum (Geraniaceae). Am. J. Bot. 90: 226-234.
Asikainen E., Mutikainen P. (2005) Preferences of pollinators and herbivores in gynodioecious Geranium sylvaticum. Ann. Bot. 95: 879-886.
Agren J., Willson M.F. (1991) Gender variation and sexual differences in reproductive characters and seed production in gynodioecious Geranium maculatum. Am. J. Bot. 78(4): 470-480.
Hill M.O., Mountford J.O., Roy D.B., Bunce R.G.H. (1999) Ellenberg's indicator values for British plants. ECOFACT, Technical Annex. Vol. 2.
Nilsson E., Agren J. (2006) Population size, female fecundity, and sex ratio variation in gynodioecious Plantago maritima. J. Evol. Biol. online early. [cited 14 May 2006]. Available from URL: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1420-9101.2005.01045.x
Ramula S., Mutikainen P. (2003) Sex allocation of females and hermaphrodites in the gynodioecious Geranium sylvaticum. Ann. Bot. 92: 207-213.
R Development Core Team. (2004) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
Waples R. S., Gaggiotti O. (2006) What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Molecular Ecology 15: 1419-1439.
Williams C.F., Kuchenreuther M.A., Drew A. (2000) Floral dimorphism, pollination, and self-fertilization in gynodioecious Geranium richardsonii (Geraniaceae). Am. J. Bot. 87(5): 661-669.