There are two methods of describing plant associations of the lake-shore: visual and analytical. The visual method is based on the simple observation of plant species within certain area. This method isnít objective and exact. Thatís why we have been chosen the analytical method as more effective one.The core of this method is the following: the lake-shore is divided into sample sites of 20 meters long, 1 meter away from shore line and 5 meters to the center of the lake. The abundance of plants species is registered for each site.
We described 53 sample sites on the Moldino lake (Tverskaya region, Udomelskij distrikt), 45 sites on the Blizhnee lake and 34 sites on the Dolgoe lake (Northern Kareliya, Loukskij district). The data were placed into e-table: species in the rows and sample sites in the columns. Data analysis was carried out in statistic environment R, version 2.0.1 (R Development Core Team, 2004). We analyzed each lake separately with help of cluster analysis (Manchattan distance, Ward method). We used plant species, that are present on more than 90% of all sites of the current lake to exclude incidental species for construction of the dendrogramm. Besides, cluster analysis only for common plant species was performed. Groups of plant species were united into clusters if the distance between the neighboring groups is less then 50 units.
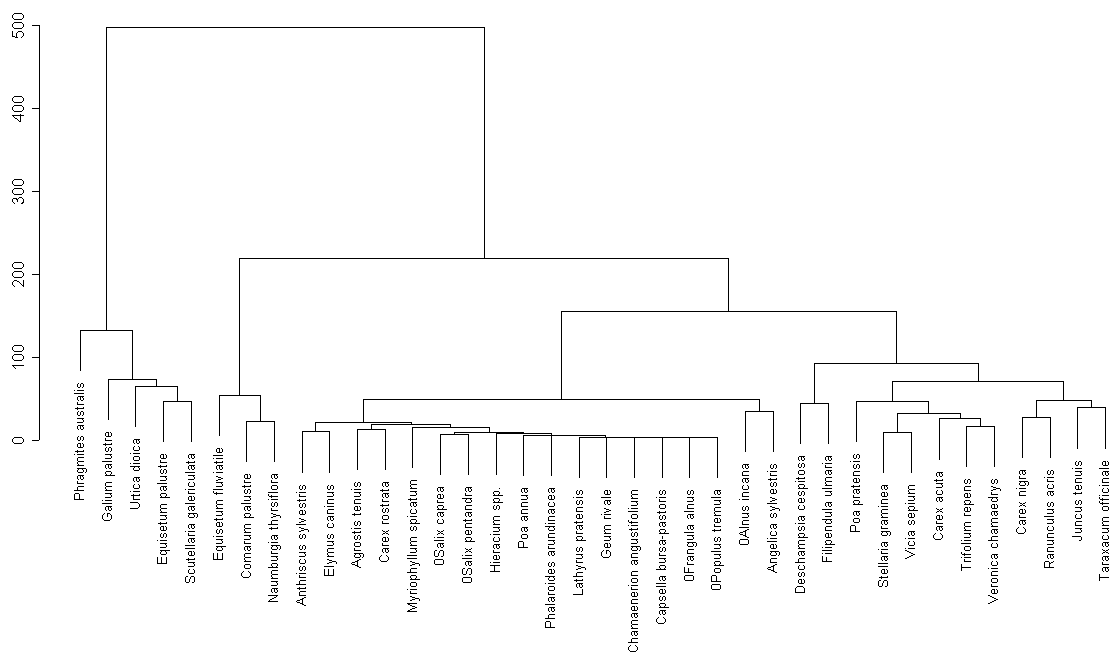
Cluster analysis only for common plant species (fig. 1) reveals the following groups:

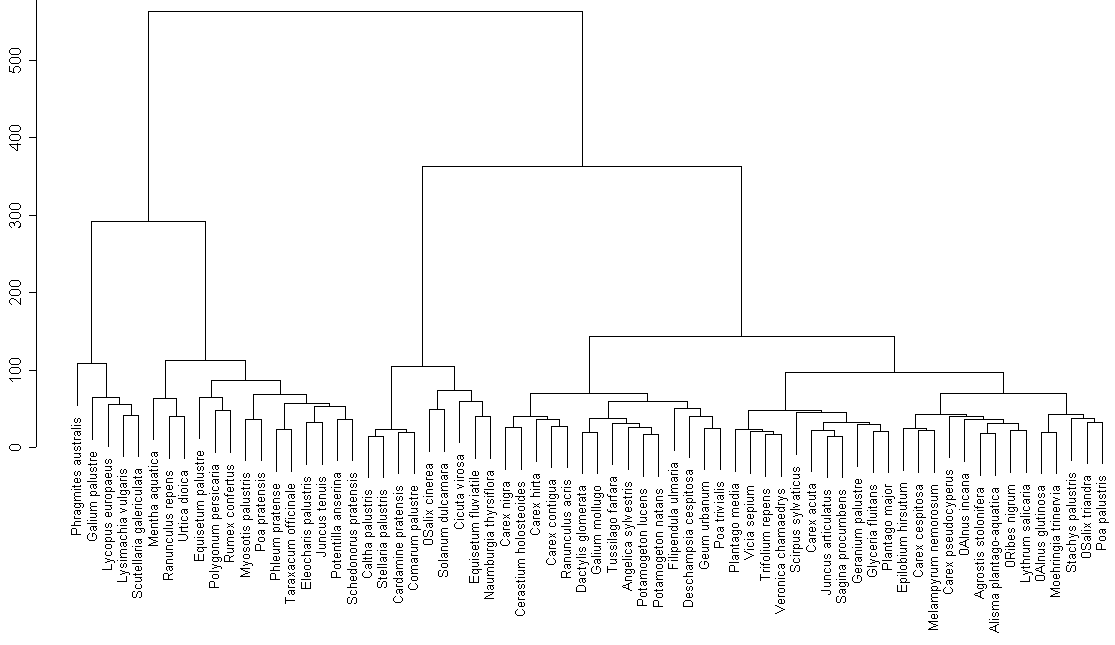
Cluster analysis of the whole species list (fig. 2) revels an obvious group that is similar to group 1. It is possible to find quite natural groups of species (for example, near-water nitrophilous species (Mentha aquatica, Ranunculus repens, etc.) or marsh species (Caltha palustris, Stellaria pallustris). On the other hand, one can find less natural groups, for example meadow species that are mixed with typical water species (pondweed is in the group with Dactylis glomerata).
Cluster analysis only for common plant species (fig. 3) reveals the following groups:
We can observe certain similarities between two lakes (groups 1, 2, 6, 7 contain common species). On the other hand there are no groups similar in their species structure.
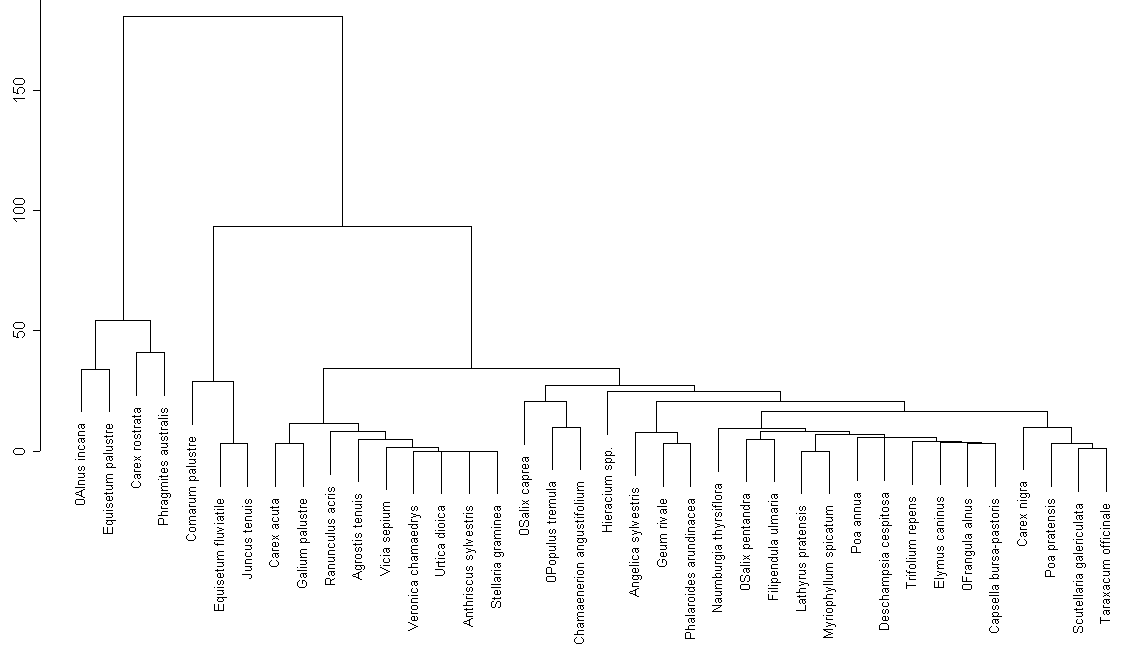
Cluster analysis of the whole species (fig. 4) list revels analogous groups, most of which can be specified as ecological (for example, group of Ledum palustre near lakes in fir-woods) or large cluster of nemoral species of moist aspen-wood and birch-wood (group of Geranium sylvaticum) and so on. There are less species of water plants on the Dolgoe lake, comparing to the Moldino lake.
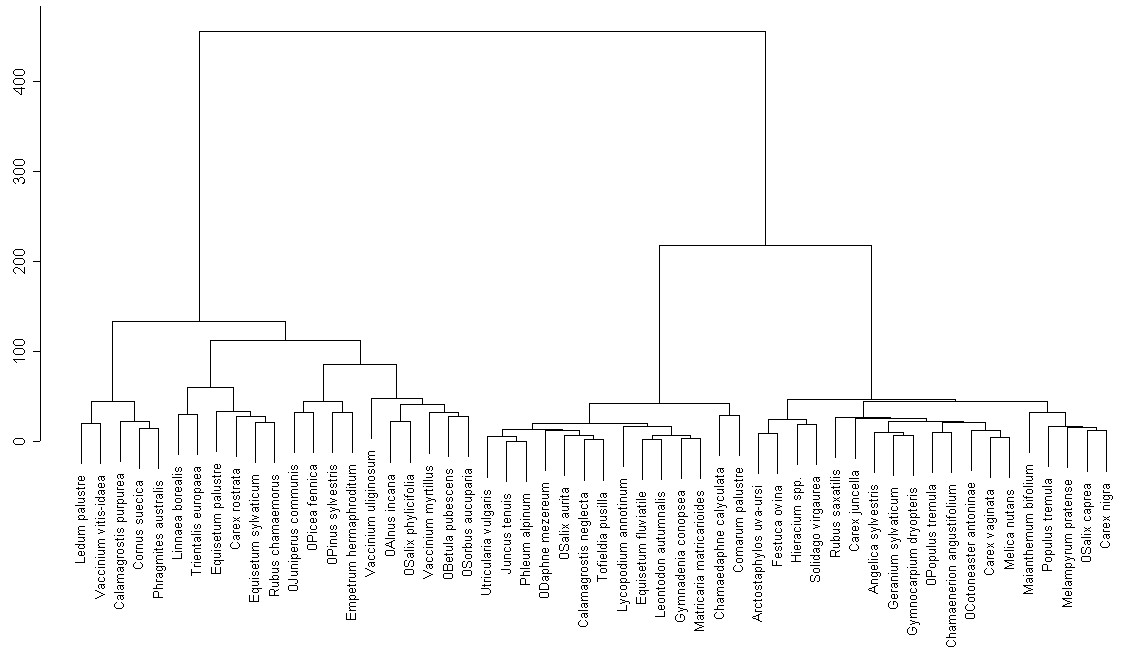
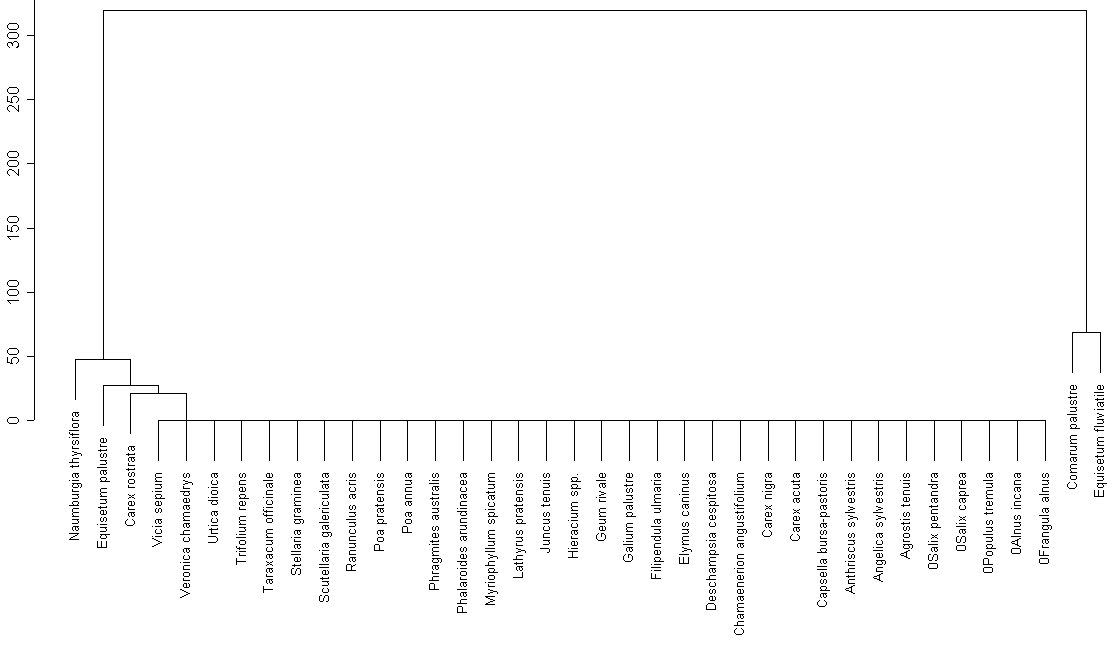
All common species on this lake form one multitudinous cluster (fig. 5), except for Comarum palustre and Equisetum fluviatile (the same group of species reveled by cluster analysis for common species from other lakes). Perhaps, these differences in species structure were caused by marsh shoreline of this lake, contrasting with shores of two other lakes.
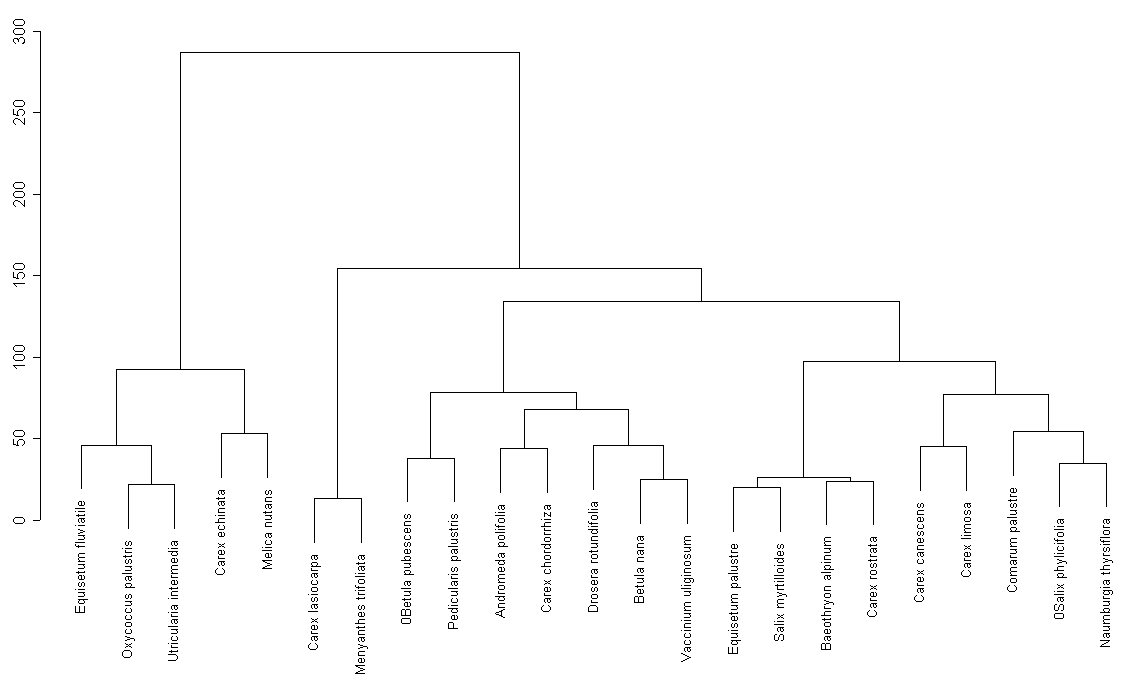
In the cluster analysis of the whole list of plant species (fig. 6) we can also mark out special groups (Carex lasiocarpa and Menyanthes trifoliata) that are typical for marsh shoreline of Karelian lakes. Most of these groups have good ecological foundation. That can be explained by the fact that we investigated the whole shoreline of this lake.
The results of cluster analysis (dendrogramms) show that our analytical method allows us mark out plant associations. For example, Moldino marsh plant group contains such species as Caltha palustris, Stellaria pallustris, Equisetum fluviatile, Comarum palustre. However, same plant associations of different lakes do not match with each other. Two of three investigated lakes (Blizhnee and Dolgoe) belong to the same latitudinal zone and one of them (Moldino) is situated far from these two lakes. Differences between these two lakes are quite large, so the structure of shore-line flora depends more on local characteristics of these lakes (perhaps on depth, characteristics of the bottom and so on), then on latitudinal zone.
Chernov V.N., Chernova E.P. Flora of the karelian lakes. Petrozavodsk, 1949. 162 p. [in Russian]
Lisitsina L.I., Papchenkov V.G. Flora of russian reservoirs: guide-book of vascular plants. M., 2000. 237 p. [in Russian]
R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. 2004.
Rasponov I.M. Vascular aquatic plants of the large lakes of North-West of USSP. L., 1985. 197 p. [in Russian]
Rossolimo L.L. Materials of Limnologycal Station in Kosino (Additional publication I). M., 1938. 205 p. [in Russian]
I would tender thanks to Eliseeva E., Loginov V. and Zlobovskaya O. for help in data collection and also to all people, who helped me in this research.