 Picture
by K. Markvitchyova
Picture
by K. Markvitchyova
Plant carnivory is the way to get mineral nutrients, mainly nitrogen and phosphorus for those plants that growth on poor soils (Chandler, Anderson, 1976; Karlsson, Carlsson, 1984). Some authors (Ellison, Gotelli, 2001) mentioned that carnivorous plants' needing in organic nitrogen decrease when the structure of traps becomes simpler and aren't great for Drosera species. The role of carnivory for plants can vary from community structure and also from the availability of different nutrients in the soil (Kraft, Handel, 1991). According to R. Swamy and H. Ram (1969) and J. Small et al. (1977), the carnivory is more or less facultative for Drosera species and influence of carnivory is weak for plants' growth in natural habitats (Stewart, Nilsen, 1992).
The behaviour of trapping leaves of Drosera species while prey catching was carefully studied in laboratory more then a hundred years ago (Darwin, 1875; Hooker, 1916). The leaves of Drosera species have short tentacles in the central part of the leaf blade and long (marginal) tentacles on its periphery.
 Picture
by K. Markvitchyova
Picture
by K. Markvitchyova
When future prey comes on the leaf blade, all tentacles bend on and cover it with slime, containing digesting enzymes.
 Picture
by K. Markvitchyova
Picture
by K. Markvitchyova
We can see (1) rapid moving of tentacles (in 10-30 sec after touching) and (2) slow moving of those tentacles that weren't in touch with prey in several hours after catching (Hooker, 1916; Bopp, Weber, 1981). B. Juniper et al. (1989) considered that sundews secrete slime just the prey is captured. The most intensive slime secretion by D. rotundifolia trapping leaves is noticed on the second day after capturing the prey (Muravnik, 2002). The edge of the leaf blade slowly bends after slime secretion and covers captured prey. When the digestion is ended, the leaf blade unwraps, the tentacles unbend and the slime dries (Hooker, 1916).
 Picture
by K. Markvitchyova
Picture
by K. Markvitchyova
However, the described mechanism of animal trapping apparently is not very reliable because, for example, the arbitrary liberation of animals from the leaf traps of D. filiformis was noticed many times (Gibson, 1991; Zamora, 1999) and ant's kleptoparasitism (Watson et al., 1982; Bingham, Cohen, 2001, our investigations) also exist.
After all, the behaviour of carnivorous plant traps depends not only on the number and condition of the captured animals. It is known, for example, that secretion activity of tentacles on the leaf blade of D. capillaris is highest while the relative air humidity is high and light intensity is low (Gomez, 1998). N.Ostashova (2002) has found that the leaves of D. rotundifolia move rhythmically without any connection with prey trapping. In the north of Karelia the period such moving was equal to 15 hours and in Vologda region (Middle Russia) it was equal to 12 hours.
Unfortunately, most observations on Drosera trapping leaves' behaviour were made in laboratories. This doesn't let us take into account natural fluctuations of number of available preys and also the influence of weather factors on the behaviour of trapping leaves. Previously we investigated different aspects of D. rotundifolia biology in natural habitats (Volkova et al., 2001; Volkova, 2002a; Volkova, 2002b; Volkova, Shipunov, 2002; Volkova et al., 2002; Volkova et al., 2003).
The main goal of the current paper is the investigation of the behaviour of D. rotundifolia trapping leaves in natural habitats and clarifying the influence of weather conditions and captured prey.
We observed: (1) two plants D. rotundifolia in Loukhi district of North Karelia, cape Ivanov Navolok (66 20' N., 33 20' I.) from 25 to 27 of July 2000, named ``series 1'' below, and (2) two plants D. rotundifolia in Vyshnevolotsk district of Tver' region (Middle Russia), western shore of the Ol'shevo lake (58 15' N., 34 30' I) from 20 to 23 of June 2002, named below as ``series 2''. The daylight time on 29 July in series 1 lasted 19 h 17 min (without dark time at all) and on 29 June in series 2 -- 18 h 17 min (these data are from astronomical software ``XEphem'', Downey, 2000).
The plants were selected randomly from typical for current natural habitat populations. Continuous observations on each pair of investigated plants lasted 72 hours in natural conditions. We estimated: (a) the shape of the leaf blade, (b) the degree of slime secretion and (c) ``bending degree'', i.e. part of curved margin tentacles (see Table 1) for each of 16 investigated leaves (see Table 2) and also counted (d) the number of captured preys.
Table 1. Criteria of visual estimation of the leaf blade's conditions
| points | degree of secretion ("wet") | shape of the leaf blade ("shape") | degree of bending ("tent") |
| 0 | poor (the leaf blade is almost dry) | ... | there is almost no bent tentacles |
| 1 | medium | almost flat | the minority of tentacles is bent |
| 2 | high (moisture is collected to the drops) | flexed | approximately a half of tentacles is bent |
| 3 | ... | bent | the majority of tentacles is bent |
| 4 | ... | ... | almost all tentacles are bent |
Table 2. Distribution of investigated leaves on the plants
| series number | number of investigated leaves (pc) | |
| on the first plant | on the second plant | |
| 1 | 5 | 4 |
| 2 | 3 | 4 |
The observations were made once in 40 minutes in series 1 and once in 30 minutes in series 2 during all period of observations. In series 2 we also measured atmospheric pressure once in two hours, air temperature and relative air humidity by aspiration Assman's psychrometer. As usually most of investigated leaves from one plant demonstrated similar behaviour (see below), for each of investigated plants we calculated "average leaf shape", "average degree of secretion" and "average bending degree" as average of the characteristics from all leaves of each plant during one observation.
We calculated nonparametric Spearman's correlation coefficients for exposing a connection between investigated characteristics of the leaf blade, weather conditions and number of captured animals (see Table 3, 5). For calculations we used STATISTICA(tm) software (StatSoft, Inc., 1999).
We didn't notice any reaction of the leaf blade to the prey during an hour after it was captured (Table 4).
Table 4. The leaf behaviour in series 1: the part of leaves with noticed changes (%)
| characteristics of leaf blade condition | change of leaf blade condition after prey came to the leaf blade in ... | change of the condition without any prey on the leaf blade | change of the condition with constant number of animals on the leaf blade | the different from the known before leaf behaviour | ||
| less then 1 h | 1--2 h | 3 h | ||||
| degree of secretion | 0 | 0 | 17 | 67 | 63 | 88 |
| shape | 0 | 17 | 17 | 83 | 100 | 25 |
| degree of bending | 0 | 50 | 50 | 100 | 75 | 50 |
Only after 2-3 hours the bending degree was increased on all investigated leaves. We didn't notice any changes in the degree of secretion as a response to the caught prey. Leaf shape was significantly changed after catching only in two leaves (~ 22%). Every investigated leaf blade frequently changed its condition when there weren't any captured prey on it or the number of captured animals was constant. All investigated leaf blades periodically dried when they had captured undigested prey and the margin tentacles of half of investigated leaves weren't bent in such a case and one quarter of leaf blades weren't curved at all. Thus, we didn't notice any significant relation between different characteristics of leaf blade condition for all investigated leaves (Fig. 1-3, Table 3).
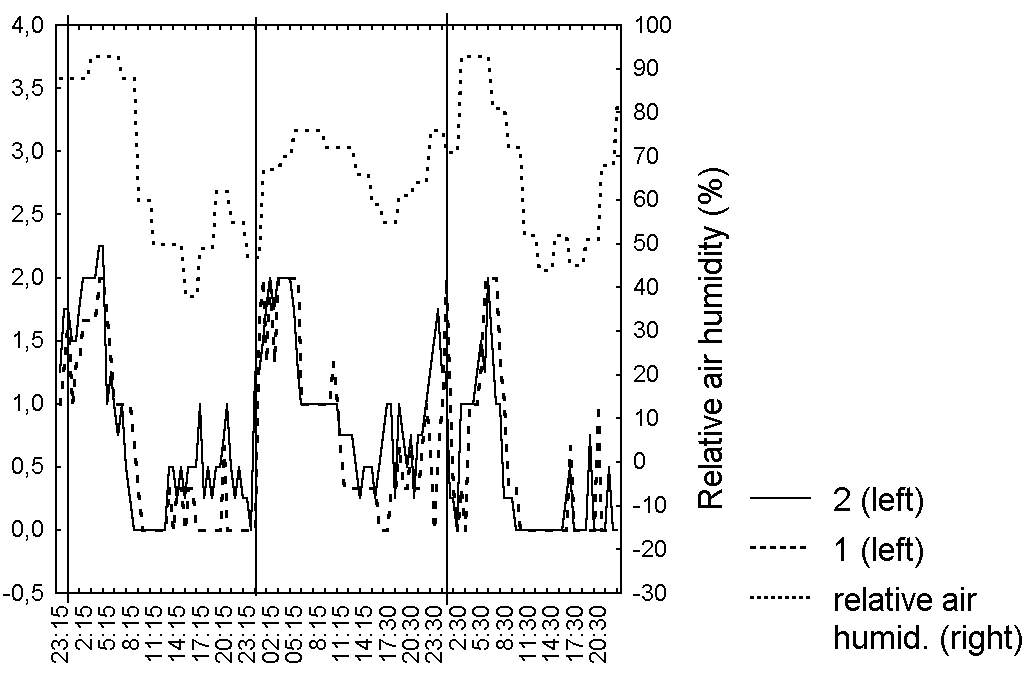
Figure 1. Changes in the degree of secretion of D. rotundifolia leaves in the Polar region
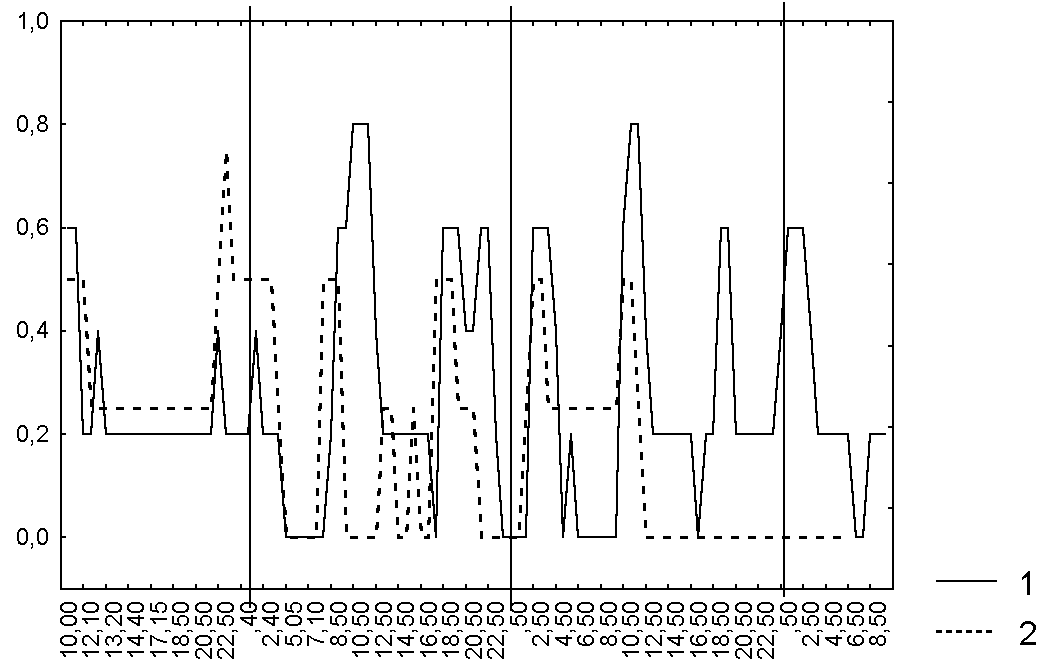
Abbreviations: For the points of leaf blade condition see Table 1. Vertical solid lines mark 00:00 of each day of observations. 1 the first investigated plant of D. rotundifolia, 2 the second investigated plant of D. rotundifolia.
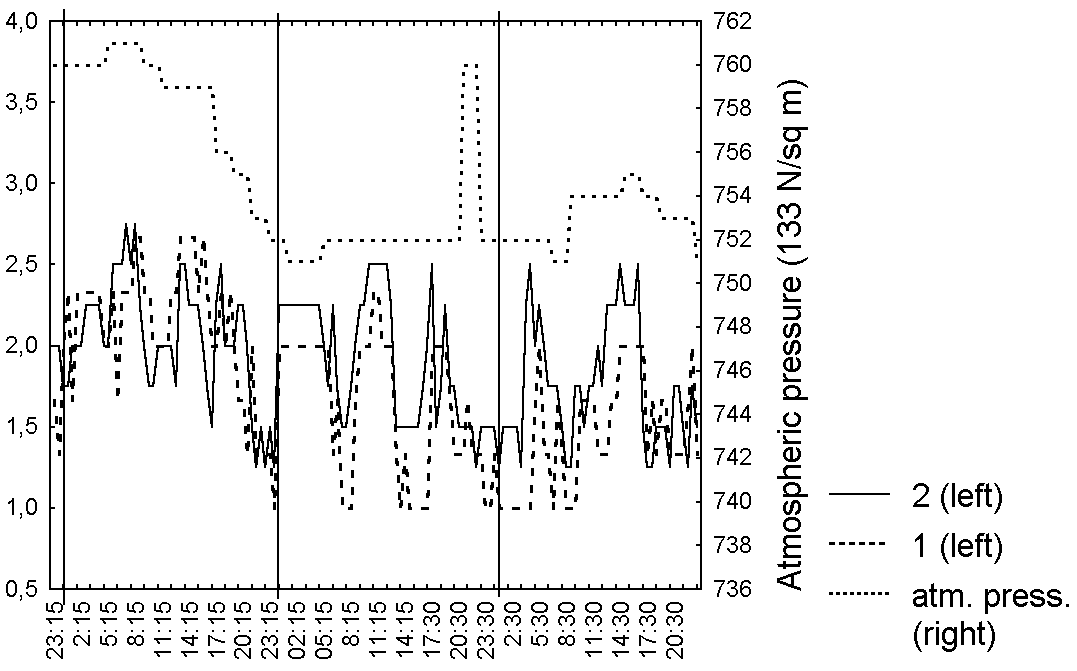
Figure 2. Changes in the shape of the leaf blade of D. rotundifolia in the Polar region
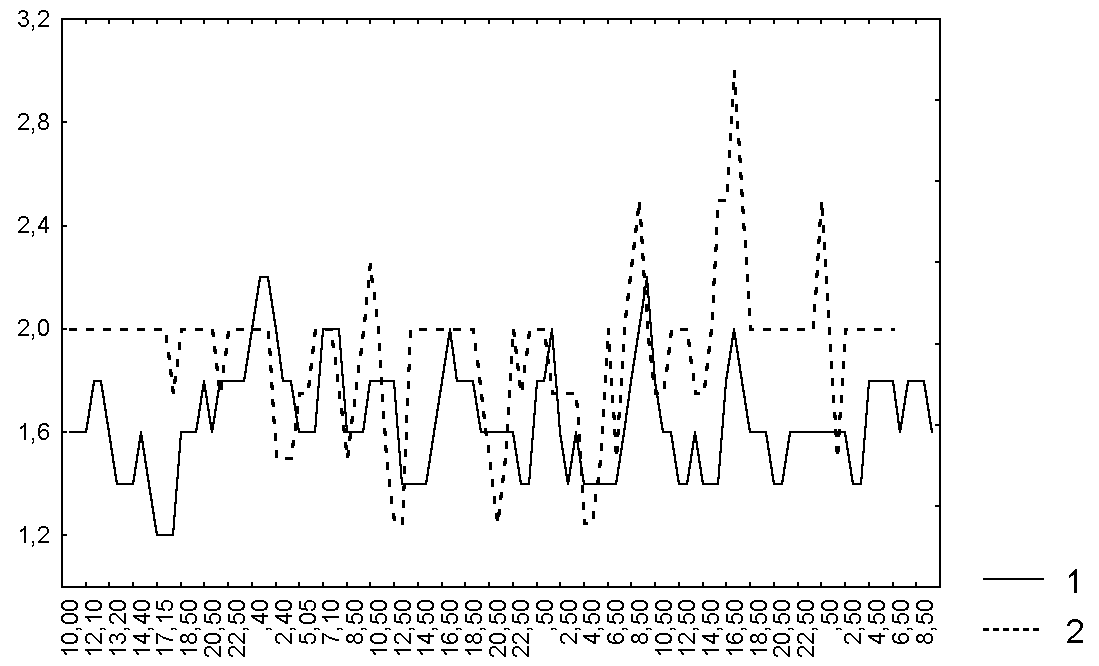
Abbreviations: see fig. 1
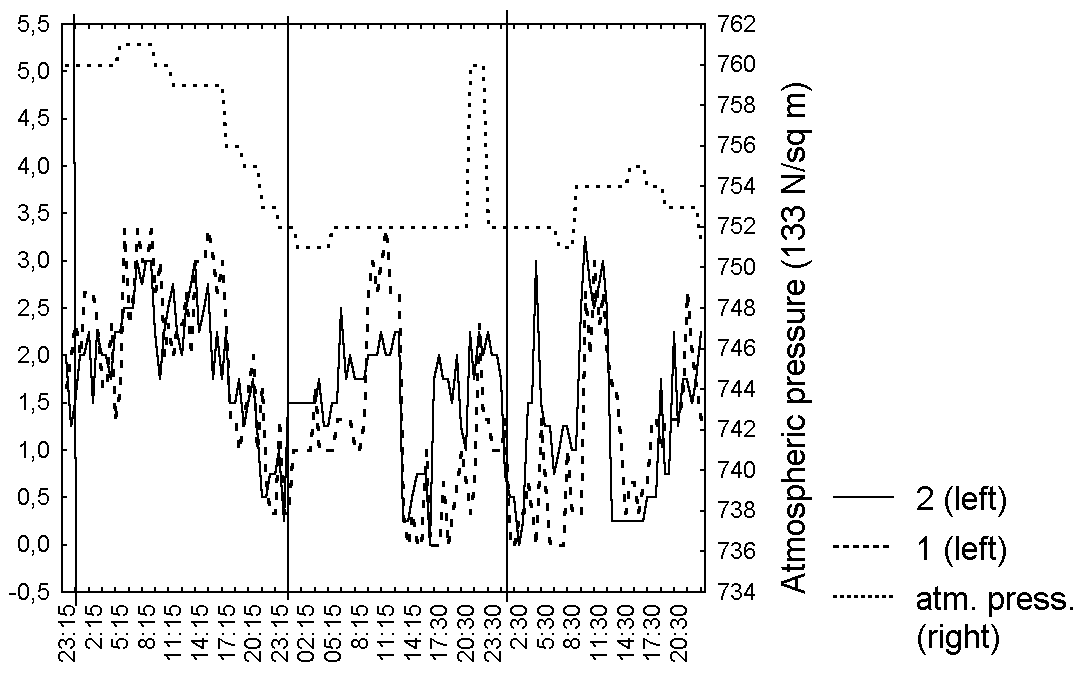
Figure 3. Changes in bending degree on the leaf blade of D. rotundifolia in the Polar region
Abbreviations: see fig. 1
Table 3. Spearman correlation coefficients between averaged characteristics of each plant's leaves (N=81, all significant correlation coefficients are included, "wetN av" -- average ("av") on all leaves of plant number N degree of leaf blade's moistening), series 1
| correlating characteristics | correlation coefficient |
| tent1 av & tent2 av | 0,59 |
| wet2 av & tent2 av | -0,54 |
| tent1 av & wet2 av | -0,33 |
| wet2 av & shape2 av | -0,25 |
For all leaves of each plant degree of secretion, leaf shape and bending is related (R=0,4-0,7, p<0.05) to averaged characteristics. Consequently, average characteristics of the leaf blade are able to describe the common tendencies of leaf behaviour of the current plant. Moreover, the changes of different characteristics of leaf blade of different plants are coincided too.
Approximately one half of investigated leaves reacted to the captured animal less then in an hour after its capturing (Table 6).
Table 6. Leaf behaviour in seria 2: the part of leaves with noticed changes (%)
| characteristics of leaf blade condition | change of leaf blade condition after prey came to the leaf blade in ... | change of the condition without any prey on the leaf blade | change of the condition with constant number of animals on the leaf blade | the different from the known before leaf behaviour | ||
| less then 1 h | 1--2 h | 3 h | ||||
| moistening | 43 | 0 | 70 | 100 | 83 | 86 |
| shape | 57 | 43 | 29 | 100 | 67 | 29 |
| condition of margin tentacles | 71 | 29 | 29 | 100 | 83 | 71 |
In this time period 40% of leaves moistened their leaf blade, 50% curved their leaf blade and 70% increased the tentacles' bending. Almost all leaves that haven't changed their condition less then in an hour after catching did so 2-3 hours after. From the one hand, for 60-70% of investigated leaves the degrees of secretion and curving depend directly from the number of caught preys. From the other hand, all leaves without any prey nonperiodically changed their characteristics for 1-6 hours.
 Picture
by K. Markvitchyova
Picture
by K. Markvitchyova
Such a behaviour was noticed for the majority (70-80%) of investigated leaves when constant number of undigested preys was mentioned. Very often we observed completely different (from described in literature) behaviour of leaves with fresh animals: nearly all investigated leaves didn't secrete slime while capturing preys
 Picture
by K. Markvitchyova
Picture
by K. Markvitchyova
and margin tentacles of 70% of investigated leaves with undigested animal on them weren't bent (Table 6).
 Picture
by K. Markvitchyova
Picture
by K. Markvitchyova
The shape of the leaf blade of all investigated leaves was related (R=0,4-0,6, p<0,001) with the bending degree (Table 5).
Table 5. Spearman correlation coefficients between averaged characteristics in seria 2 (N=144, all significant correlation coefficients are included, abbreviations as in Table 3)
| correlating characteristics | correlation coefficient |
| wet2 av & wet1 av | 0,78 |
| tent2 av & tent1 av | 0,65 |
| shape2 av & shape1 av | 0,64 |
| shape1 av & tent1 av | 0,61 |
| tent2 av & shape1 av | 0,41 |
| shape2 av & tent1 av | 0,36 |
| shape2 av & tent2 av | 0,34 |
| wet2 av & shape2 av | 0,24 |
| shape2 av & wet1 av | 0,24 |
| relative air humidity & wet1 av | 0,68 |
| relative air humidity & wet2 av | 0,61 |
| air temperature & wet1 av | -0,61 |
| atmospheric pressure & tent1 av | 0,56 |
| atmospheric pressure & shape1 av | 0,48 |
| air temperature & wet2 av | -0,46 |
| atmospheric pressure & tent2 av | 0,41 |
| air temperature & shape1 av | 0,23 |
| atmospheric pressure & wet2 av | -0,23 |
| relative air humidity & tent2 av | 0,20 |
| atmospheric pressure & shape2 av | 0,20 |
We didn't reveal any significant linkage between the degree of secretion, leaf shape and bending (Fig. 4-6, Table 5).
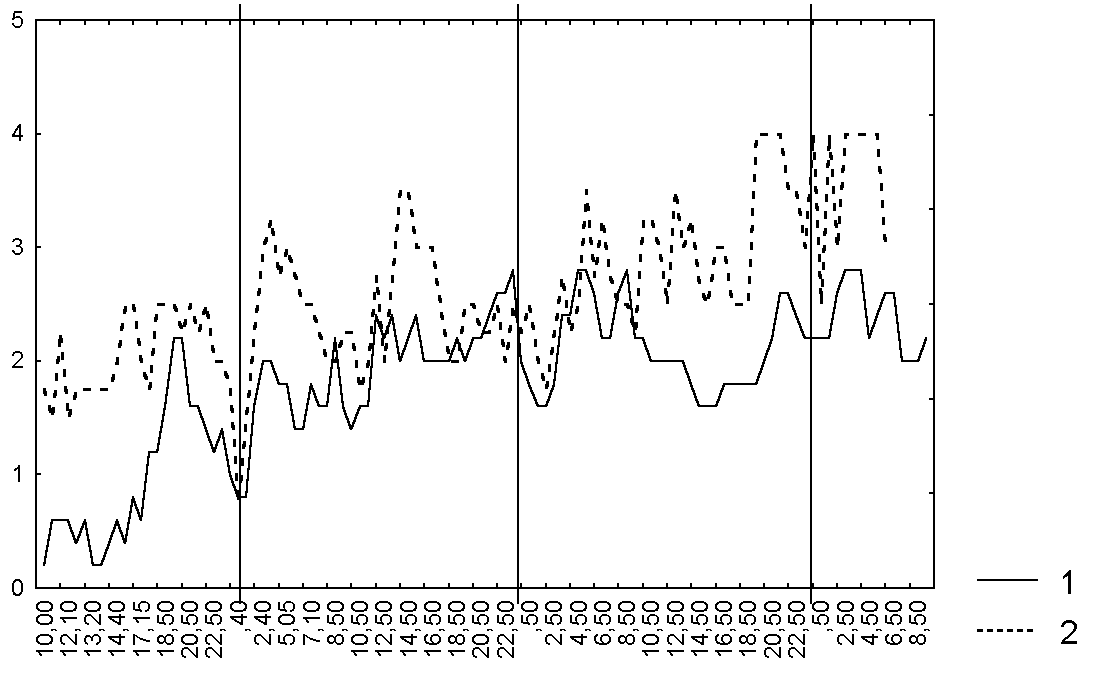
Figure 4. Changes in the degree of secretion of D. rotundifolia leaves in the Middle Russia
Abbreviations: see fig. 1
Figure 5. Changes in the shape of the leaf blade of D. rotundifolia in the Middle Russia
Abbreviations: see fig. 1
Figure 6. Changes in bending degree of D. rotundifolia in the Middle Russia
Abbreviations: see fig. 1
As in series 1, the degree of secretion, leaf shape and bending for each leaf in series 2 correlated (R=0,7-0,9, p<0.05) with averaged characteristics (Table 5). Very interesting is synchronisation in leaf behaviour for all of investigated leaves, without any dependence from belonging to one plant (Fig. 4-6, Table 5). The degree of secretion for all investigated leaves directly depends (R=0,5-0,7, p=0) on the relative air humidity (Fig. 4, Table 5). We noticed for all investigated leaves more weak positive dependence (R=0,2-0,5, p<0.05) between bending degree and leaf shape, from one side, and the atmospheric pressure (Fig. 5, 6, Table 5) from another. However, we didn't observe any dependence of the captured prey number from the weather conditions.
The air temperature in the observation period remained constant, and its average meaning was 17 C. Daily fluctuations of the air temperature were clearly shown: the temperature minimum was observed at 5-6 a.m., and was equal to 9 C, the maximum was observed at 2-3 p.m., being equal to 26,4 C. We didn't mention significant influence of air temperature on characteristics of the leaf blade. Weak dependence of characteristics of the leaf blade on air temperature, observed for some leaves are definitely caused by the clear negative linkage between the air temperature and its relative humidity.
Our observations show that different characteristics of the leaf blade of D. rotundifolia change independently from each other and from the presence of the prey. This is typical for Middle Russia and for the polar regions as well. Changing of the different leaf characteristics was more co-ordinated in series 2 than in series 1. Moreover, the leaf reactions to the capturing were observed more clearly in series 2 too. Such differences are probably connected with harder habitat conditions for D. rotundifolia in the polar region (in compare with temperate Middle Russia) what could influence negatively to the activity of carnivorous behaviour for the sundews from northern populations.
The fact of co-ordinated behaviour of leaf blades of different plants also indirectly shows the absence of clear dependence of trapping leaves behaviour on presence of prey on them. This fact was investigated by field observations of N. Ostashova (2002) too.
The degree of leaf blade's moistening for carnivorous plants as for all other plants cannot be independent from the relative air humidity that was proved by L.D. Gomez (1998) for D. capillaris. The chemical composition of slime may change as a response of catching while the amount of the slime is practically constant. The relation of leaf blade's shape and bending to the atmospheric pressure was probably caused by the dependence these leaf blade characteristics from the in-cellular pressure. The laboratory observations on the trapping leaves behaviour (Darwin, 1875; Hooker, 1916; Muravnik, 2002) couldn't take into account the influence of weather conditions on the leaf blade conditions, which is seemed to exceed the effect of captured prey in natural conditions.
We think that the changing of the leaf blade condition of D. rotundifolia can be accidental and are only corrected by the external factors such as relative air humidity, atmospheric pressure and presence of the prey on the leaf blade. We didn't observed clear rhythm of leaf blade's behaviour that is contrary to N. Ostashova (2002) opinion.
We can consider that secretion, leaf blade curving and bending of the marginal tentacles in some time after prey capturing in most cases is a coincidence. This situation isn't unique, because the active reaction of traps on the captured animal isn't an obligatory attribute of the carnivorous plants. Some Drosera species (e.g., D. binata) have large leaves that simply cannot change its shape relatively fast and phylogenetically close Nepenthes species have pitcher-traps that not capturing the prey actively (Ellison, Gotelli, 2001). Maybe only for Aldrovanda and Dionaea capturing the prey and changing of trapping leaves' characteristics are strongly related.
Our data about the absence of clear linkage of trapping leaves behaviour and presence of captured animals on them for D. rotundifolia proof the recent hypothesis on the facultative role of carnivory for so-called ``carnivorous plants'' (Dore Swamy, Maham Ram, 1969; Small et al., 1977; Stewart, Nilsen, 1992; Ellison, Gotelli, 2001).
The data were collected during field practices of Moscow South-West High School, lead by Dr. S. Glagolev. We thank E. Altshuler, Dr. T. Braslavskaya, P. Buntman, V. Chava, N. Gorbunov, Ya. Kosenko, D. Nazarov, N. Ostashova, E. Peskova, I. Pokrovskii, O. Vasiljeva and T. Volkova for essential help in field investigations and also Dr. L.E. Muravnik and A.N. Ivanova for useful comments in preparing the manuscript.
Bingham R.A., Cohen N.D. Flowering, reproduction and kleptoparasitism in an extreme southern disjunct population of Drosera rotundifolia // 2001 Meeting of the Botanical Society of America (BSA) [Electronic resource]. Mode of access: http://www.botany2001.org
Bopp M., Weber I. Studies on the hormonal regulation of leaf blade movement of Drosera capensis L. Physiologia Plantarum. 1981. Vol. 53. P. 491-496.
Chandler G.E., Anderson J.W. Studies on the nutrition and growth of Drosera species with reference to the carnivorous habit // New Phytol. 1976. Vol. 76. P. 129-141.
Darwin C. The insectivorous plants. London, 1875.
Dore S.R., Maham R.H.J. Studies on growth and flowering in axenic cultures of insectivorous plants. Phytomorphology. Vol. 19.P. 363.
Downey E.C. Xephem. Astronomy program. Version 3.2.2. 2000. [Electronic resource]. Mode of access: http://www.clearskyinstitute.com/xephem/.
Ellison A.M., Gotelli N.J. Evolutionary ecology of carnivorous plants // Trends in Ecol. and Evol. 2001. Vol. 16. N 11. P. 623-629.
Gibson T.C. Differential escape of insects from carnivorous plant traps // American Midland Naturalist. 1991. Vol. 125. N 1. P. 55-62.
Gomez L.D. Natural history and occurence of the "insectivorous plant" Drosera capillaris (Droseraceae) in Costa Rica // Revista de Biologia Tropical. 1998. Vol. 46, N 4. P. 1033-1037.
Hooker H.D. Physiological observations on Drosera rotundifolia // Bull. Torr. Bot. Club. 1916. Vol. 43. N 1. P. 1-27.
Juniper B.E., Robins R.J., Joel D.M. The carnivorous plants. London, 1989.
Karlsson P.S., Carlsson B. Why does Pinguicula vulgaris L. trap insects? // New Phytol. 1984. Vol. 97. P. 25-30.
Muravnik L.E. The influence of chemical stimulation on the ultrastructure of secretory cells in the tentacles of two Drosera species // Fiziologiya rastenij. 2002. Vol. 47, N 4. P. 614-623. [in Russian]
Ostashova N.V. Ecotopical preferences and leaf movements of Drosera species. Graduate work. Moscow State University, Biological faculty. Moscow, 2002. P. 11, 15. [in Russian]
Small J.G.C., Onraet A., Grierson D.S., Reynolds G. Studies on insect-free growth, development and nitrate-assimilating enzymes of Drosera aliciae hamet // New Phytol. 1977. Vol. 79. P. 127-133. StatSoft, Inc. 1999.
STATISTICA for Windows [Computer Program Manual]. Tulsa, OK.
Stewart C.N., Nilsen E.T. Drosera rotundifolia growth and nutrition in a natural population with special reference to the significance of insectivory // Can. J. Bot. 1992. Vol. 70. N 7. P. 1409-1416.
Volkova P. A. On the morphological differences of three sundew species (Drosera L., Droseraceae) // International scientific conference on the systematic of higher plants. Moscow, 2002a. P. 29-30. [in Rissian]
Volkova P., Kumskova E., Shipunov A. Peculiarities of insect catching by round-leafed sundew (Drosera rotundifolia L., Droseraceae) // Materials of conference of young Ukraine botanists. Lvov, 2002. P. 137-138. [in Russian]
Volkova P.A. The materials on the ecology of Drosera rotundifolia L. on the islands of Keretskii archipelago and Kiv bay // III scientific workshop of marine biological station of Saint-Petersburg state university. Saint-Petersburg., 2002b. P. 10-14. [in Russian]
Volkova P.A., Kumskova E.M., Nazarov D.Yu., Pokrovskii I.G. The connection between morphophysiological characteristics, success of insect catching and habitat conditions of different species of carnivorous plants // V Russian population workshop "Population, community, evolution". Part 1. Kazan', 2001. P. 14-17. [in Russian]
Volkova P.A., Kumskova E.M., Shipunov A.B. The dependence of morphophysiological characteristics on success of insect catching and habitat conditions for Drosera rotundifolia L., D. anglica Huds. and D. x obovata Mert. et Koch (Droseraceae) and Pinguicula vulgaris L. (Lentibulariaceae) // Bulleten MOIP. Sect. biol. 2003. Vol. 108. N 1. P. 72-78. [in Russian]
Volkova P.A., Shipunov A.B. The behaviour of trapping leaves of carnivorous herb Drosera rotundifolia L. (Droseraceae). // Ecological botany. Syktyvkar, 2002. P. 61-62. [in Russian]
Watson A.P., Mathiersseu J.N., Sprigett B.P. (1982). Arthropod associates and macronutrient status of the red-ink sundew (Drosera erythrorhiza Lindl.) Aust. J. Ecol. Vol. 7. P. 13-22.
Zamora R. Conditional outcomes of interactions: the pollinator-prey conflict of an insectictivorous plant // Ecology. 1999. Vol. 80. N 3. P. 786-795.